This post briefly explores tumour-on-chip models, starting with conventional 2D models until the arrival of more complex 3D approaches.
Introduction
Cancer continues to be one of the leading causes of death worldwide, and about one out of every six deaths is caused by this disease. According to GLOBOCAN, the number of annual cases continues to rise and is expected to increase to about 30,2 million cases by 2040 [1].
Recreating realistic models of micro-tumour niches has long been a challenge for the scientific community. Internal heterogeneity (i.e., cancer cells, stromal cells, immune cells, vascular cells), additional compounds (cytokines and growth factors) and three-dimensionality of tumours hinder efforts to understand and reproduce the tumour niche in vitro. Nowadays, less than 10% of anticancer drugs entering clinical trials are commercialised, and most of them fail on the final steps of clinical development, even after achieving positive results in preclinical trials. These facts suggest that current models are not capable of reproducing the real in vivo conditions of the tumour niche [2].
Conventional cell culture models
Conventional two-dimensional models offer a simple and cost-effective tool to study tumour biology. However, they lack on essential features to closely mimic the complex cellular environment in vivo [3]. For instance, cell-cell and cell-extracellular matrix interactions -which are responsible for differentiation, proliferation, morphology, gene expression and other functions- are altered in these type of model [4]. The importance of overcoming these limitations have led to develop more complex methods such as three-dimensional models with organoids, which better fulfill the main requirements of representing the spatial and chemical complexity of living tissues. Nevertheless, there are still difficulties to solve. The shape and size of organoids is highly variable, plus entrapped cells are difficult to quantify and visualize with microscopy techniques. Another drawback is the lack of mechanical cues and fluid flow, to which cells are normally exposed in human tissues [5].
Organ-on-chip
Microfluidic organ-on-chip models cover most of the aforementioned limitations better mimicking microstructure, dynamic mechanical properties and biochemical functionalities of whole living organs and solid tumours, as well as allowing a continuous feed of nutrients and pharmaceutical compounds [6]. Furthermore, due to its small dimensions, it is suitable for high-throughput screening as the amount of drug consumed is low [7].
The current use of Organ-On-Chip devices encompass plenty of branches within the cancer field. In addition to simulating tumour microenvironment and its characteristic regions (Fig. 1) [8], it also recreates tumour cell migration and invasion [9], metastasis modelling [10], vascularization and extravasation [11], tumour microenvironment recreations [12], immuno-oncology studies [13], drug screening [14], etc. Understanding cancer cell migration and metastasis with a controlled microenvironment on chip would help to gain new insights into cancer therapeutic strategies and to develop personalized diagnostics based on the tumour cell migration phenotype. Current developments in this field have enabled researchers to generate not only a more refined model allowing cells to migrate in 3D but also single-cell sorting and analysis of migration phenotype [15].
Tumour-on-Chip
Modelling the tumour microenvironment (TME) is also one of the most frequently studied applications. Among the TME, On-Chip models are contributing to the understanding of the activation of tumour-associated macrophages (TAMs) and how they enhance tumour invasion [16]. Another great advantage of using microfluidic technologies comes from tumour-immune studies, providing insights into the crosstalk between tumour and immune cells [17,18].
The rising importance of microfluidic platforms to precisely mimic tumour environments has led to the development of several successful models, which are minimising the previous gap between in vitro and in vivo studies, thus bringing personalized medicine applications closer to practice.
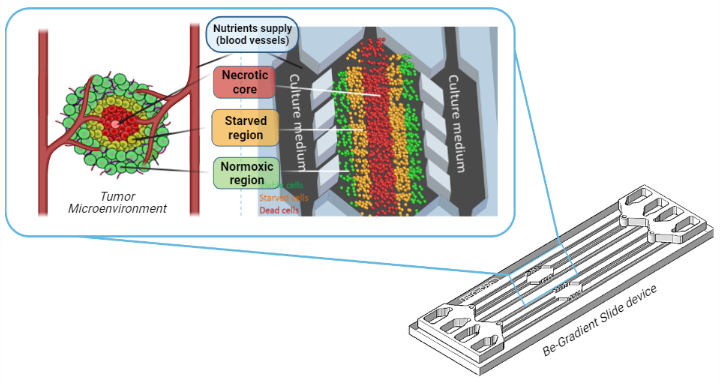
Figure 1. The figure shows a device fabricated by the company Beonchip, called Be-Gradient. It consists of a central chamber where tumour cells are cultured in a 3D matrix, creating a tumour-like structure with a central necrotic core (red cells) surrounded by a starved region (orange) and an external region of viable cells (green cells). The central chamber is in contact with two lateral channels, which are meant to simulate blood vessels, in order to supply nutrients to tumour cells.
Authors
This article has been written by Clara Bayona and Sara Abizanda.
Bibliography
- Global Cancer Observatory (https://gco.iarc.fr/).
- Trujillo-de Santiago, G. et al. Materials 12, 2945 (2019).
- Duval, K. et al. Physiology 32, 266–277 (2017).
- Kapałczyńska, M. et al. Arch. Med. Sci. AMS 14, 910–919 (2018).
- Bhatia, S. N. & Ingber, D. E. Nat. Biotechnol. 32, 760–772 (2014).
- Huh, D., Hamilton, G. A. & Ingber, D. E. Trends Cell Biol. 21, 745–754 (2011).
- Beißner, N., Lorenz, T. & Reichl, S. Organ on Chip. in Microsystems for Pharmatechnology (ed. Dietzel, A.) 299–339 (Springer International Publishing, 2016).
- Ayuso, J. M. et al. Sci. Rep. 6, 36086 (2016).
- Um, E., Oh, J. M., Granick, S. & Cho, Y.-K. Lab. Chip 17, 4171–4185 (2017).
- Caballero, D. et al. Biomaterials 149, 98–115 (2017).
- Chen, M. B., Whisler, J. A., Jeon, J. S. & Kamm, R. D. Integr. Biol. 5, 1262 (2013).
- Wan, L., Neumann, C. A. & LeDuc, P. R. Lab. Chip 20, 873–888 (2020).
- Kumar, V. & Varghese, S. Adv. Healthc. Mater. 8, 1801198 (2019).
- Rodriguez, A. D. et al. Lab. Chip 20, 1658–1675 (2020).
- Schwarz, J. et al. Sci. Rep. 6, 36440 (2016).
- Zhao, Y. et al. Oncotarget 6, 39196–39210 (2015).
- Hsu, T.-H. et al. Integr Biol 4, 177–182 (2012).
- Hachey, S. J. & Hughes, C. C. W. Lab. Chip 18, 2893–2912 (2018).

