Summary
This guide summarizes Beonchip’s microfluidic chip design customization options to improve your cell culture model. It highlights the importance of channel dimensions in microfluidic chips for key aspects such as cell culture area, microscopy inspection, and shear stress optimization. Additionally, it addresses the significance of pore size for common biological applications.
If you wish to further customize your microfluidic chip—such as integrating your own membrane, altering channel shapes, or modifying features unavailable on our website—please don’t hesitate to contact us.
Microfluidic Chip Design Customization: Channel Width
Influence on Cell Culture Area
The channel width of microfluidic devices designed for cell culture impacts the surface area available for cell seeding. Wider channels provide larger cell culture areas than narrower channels. For example, our standard channel width of 1.5 mm in devices such as the Be-Flow provides an area of approximately 66 mm2. Expanding the channel width to 2.5 mm increases the area to 99 mm2, representing a 50% increase over the standard dimension.
This increase in surface area supports experiments requiring higher cell concentrations to provide sufficient genetic material for downstream assays such as PCR. Conversely, researchers may use narrow channels as an effective alternative when studying less proliferative cell types or those requiring low passage numbers and/or expensive culture medium.
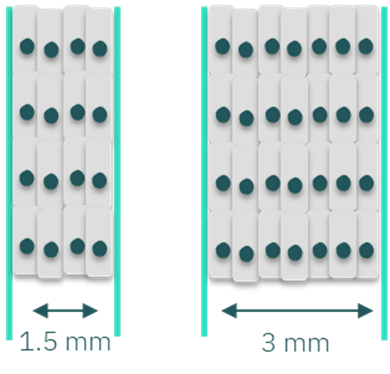
Figure 1: Scheme of the difference in cell density by customizing the channel’s width. Higher channel width allows increased cell density.
Influence on Shear Stress
Channel width also influences the shear stress experienced by cells when a perfusion system is connected. Shear stress (Ʈ) is a mechanical force generated when a tangential force (F) acts on a surface (area = A).
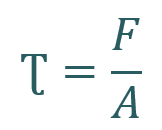
For a Newtonian fluid, shear stress depends on the viscosity (η) and flow rate (Q). Given that the viscosity remains constant as that of the cell culture medium, the width (w) and height (h) of the channels are critical factors affecting shear stress.
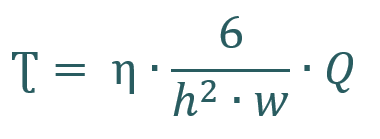
Why is the flow rate a limiting factor in your experimental setup?
Cells in native tissues experience varying shear stress levels. Researchers determine the optimal range to replicate tissue conditions in vitro. They adjust shear stress by modulating the flow rate, which directly influences it.
However, high flow rates may limit prolonged cultures due to significant media volumes required, especially without recirculation. This necessitates careful consideration of media usage and system design to ensure effective and sustainable cell culture conditions.
Researchers can adjust channel dimensions to minimize the volume of cell culture medium needed while applying high shear stress. Using the same flow rates, narrower channels generate higher shear stress than wider ones.
Another limiting factor related to the flow rate is the precision range of the flow control system. For equipment with limited flow rate, adjusting channel dimensions may be a cost-effective alternative to buying new equipment for desired shear stress.
Microfluidic Chip Design Customization: Channel height
Influence on Shear Stress
The height of the channel significantly affects shear stress, having twice the impact compared to the channel width. For instance, to achieve a desired shear stress of 0.02 dyn/cm2 in a channel with a standard height of 375 µm, a flow rate of 5.8 µL/min is required. If the channel height is reduced by half to 188 µm, the necessary flow rate decreases to 1.47 µL/min, resulting in a 3.9-fold reduction in media volume.
As mentioned above, the precision range of the perfusion system might also be a limiting factor that requires attention when choosing the best channel height.
Influence on Microscopy Analysis
In devices with vertically stacked channels, such as our Be-Transflow and Be-Doubleflow, the height of the channels, particularly the bottom channel, is crucial for microscopy compatibility. High-magnification lenses (40x, 60x, 100x) have short focal distances (<200 µm), complicating imaging of top membrane-seeded cells.
Under these circumstances, we recommend a custom device with a shorter bottom channel (around 150 µm) and a thinner base (100 or 50 µm), so the membrane is closer to the objective. With such dimensions, the user can perfectly capture the cells seeded on top of the membrane with the highest magnification.
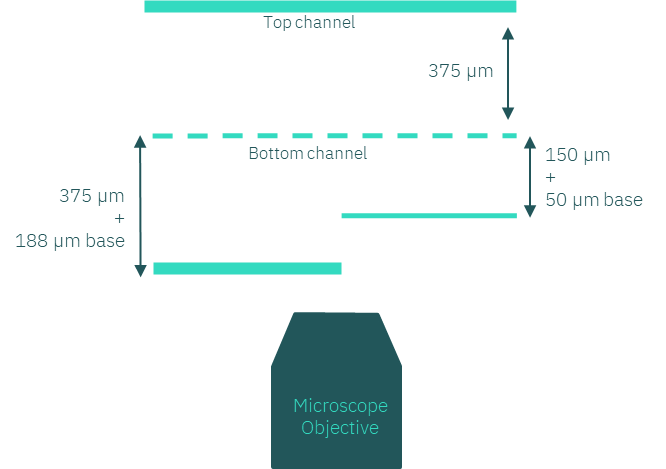
Figure 2: Scheme of different bottom channel heights concerning the focal distance of the microscope objective.
Microfluidic Chip Design Customization: Membrane’s pore size
Pore size significantly impacts cell adhesion, cell-cell interaction, and cell transmigration across the membrane, depending on the specific objectives of tissue regeneration. The initial cell adhesion to scaffold surfaces is crucial for triggering signals that promote cell proliferation and differentiation. Pore size must facilitate the formation of functional gap junctions and ensure appropriate interactions with other cells and the extracellular matrix (ECM). Membranes with pore sizes smaller than 1 µm are optimal for better cell attachment. Pore sizes between 1 and 3 µm facilitate anchorage-dependent cell-cell interactions. Membranes with pore sizes ranging from 3 to 8 µm support direct cell-cell contact, as well as cell migration and invasion [1].
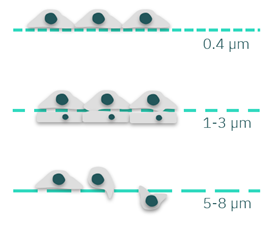
Figure 3: Scheme of cells cultured in membranes with different pore sizes. Membranes with 0.4 µm pores can be used for better cell attachment. Pores of 1-3 µm can facilitate cell anchorage and cell-cell interactions. Bigger pore sizes (5-8 µm) can be used for cell migration and invasion models.
Some examples:
- Human osteoblasts fully adhered and spread on surfaces with pores ranging from 0.2 to 1 µm. In contrast, the cells became spherical, displaying few filopodia and lamellipodia, on membranes with larger micropores (3-8 µm). Additionally, membranes with 5-8 µm pores promoted increased osteogenic differentiation, with the highest differentiation occurring at 8 µm[2] .
- Studies on co-culturing showed that pore sizes of 0.02, 0.4, 0.6, and 0.8 µm are ideal for cultivating endothelial and smooth muscle cells separately. However smooth muscle cells began to migrate across the membrane when the pore size reached 2.0 µm [3].
- Fibroblasts cultured on one side of a membrane with ~1 µm pores reached and contacted cells on the opposite side[4].
- Researchers cultured human embryonic stem cells and feeder cells on opposite sides of membranes with 1, 3, and 8 µm pores for 5 days. The results revealed that embryonic stem cells attached more effectively to membranes with 3 and 8 µm pores than to those with 1 µm pores. Membranes with pore sizes over 3 µm enabled feeder cells to migrate upwards, while 1 µm pores optimized embryonic stem cell growth without feeder cell contact. Furthermore, 3 µm pores, unlike 1 µm pores, supported extended in vitro growth of embryonic stem cells [5].
Our Be-Doubleflow and Be-Transflow devices have a porous membrane separating two compartments. The pore size for standard devices is 1 µm, which has been described as a suitable size for multiple applications, such as co-culture. In addition, we offer other membrane sizes as customizable options. The 0.4 µm pore size is usually used for co-cultures or permeability studies, with minimal cell migration. For cell migration or invasion, we offer pore sizes of 3, 5 or 8 µm which are more appropriate.
Bibliography
- Bružauskaitė, I., Bironaitė, D., Bagdonas, E. & Bernotienė, E. Scaffolds and cells for tissue regeneration: different scaffold pore sizes—different cell effects. Cytotechnology 68, 355–369 (2016), DOI:10.1007/s10616-015-9895-4.
- Lee, S. J. et al. Response of MG63 osteoblast-like cells onto polycarbonate membrane surfaces with different micropore sizes. Biomaterials 25, 4699–4707 (2004), DOI:10.1016/j.biomaterials.2003.11.034.
- Saunders, K. B. & D’Amore, P. A. An in vitro model for cell-cell interactions. Vitr. Cell. Dev. Biol. 28, 521–528 (1992), DOI:10.1007/BF02634136.
- Kim, M. Y., Li, D. J., Pham, L. K., Wong, B. G. & Hui, E. E. Microfabrication of high-resolution porous membranes for cell culture. J. Memb. Sci. 452, 460–469 (2014), DOI:10.1016/j.memsci.2013.11.034.
- Kim, S. et al. A Novel Culture Technique for Human Embryonic Stem Cells Using Porous Membranes. Stem Cells 25, 2601–2609 (2007), DOI:10.1634/stemcells.2006-0814.

