Introduction
In our previous technical note “Be-Doubleflow App. notes: Gut-on-chip 1” we explored gut-on-a-chip (GOC) model using Be-Doubleflow device in collaboration with AINIA. There we compared our device with an insert platform with GOC outperforming the control system by expressing differentiation markers earlier and with increased expression of mucins and defensins. Following that work, we complemented the studies performed and deliver a detailed note with the protocols and results.
To better mimic the intestine, GOC must present adequate intestinal barrier function. Researchers use absorption studies to assess the entrance and permeation of substances, such as nutrients or drugs [1]. Within these studies, digestion blanks serve as control samples to compare with the digested ingredients or food of interest. The digesta contains digestive enzymes and bile salts at concentrations that, although physiologically relevant, can harm cells. Therefore, various strategies exist to detoxify this digesta, one of which is dilution with culture medium [2]. However, extensive dilution of the sample complicates subsequent analysis.
This guide compares the Be-Doubleflow (BDF) device as GOC dynamic system with static culture for the set-up parameters for absorption studies using a digestion blank.

Materials
1. Be-Doubleflow device

Figure 1 Be-Doubleflow device and cell culture scheme in the device channels.
Be-Doubleflow (BDF) design (Figure 1) consists of two perfusable channels connected via a PET porous membrane. This device is suitable to study the crosstalk between different 2D and 3D cultures in a biomimetic environment and control the efficiency of the interaction by selecting the optimal pore size for specific applications. It is optimal when gas control is needed, for studying the effect of circulating particles (bacteria, immune system, circulating tumour cells) and for epithelium/endothelium barrier models when flux plays a role in both sides of the co-culture. In this work, the upper/apical channel is destined for intestine lumen and the lower/basal channel as endothelial vessel.

* The volumes presented in the table are theoretical values calculated for the standard products. Changes in the device features of custom chips may modify the exact channel volume.
2. Fluigent’s Equipment

Figure 2. Positive controllers
Fluigent® positive controllers precisely regulate the flow rate of a microfluidic system by applying small amounts of compressed air over a closed reservoir to induce the rise of a solution through an output tube (Figure 2).
- Air pressure source. This can be a compressed air intake or external compressor. The use of the external pressure compressor will facilitate the assembly of systems with cell culture in sterile conditions working with a biosafety cabinet. Once the system is assembled and transported to the incubator, it can be connected to a fixed compressed air intake.
- Controller. Regulates pressure input to FlowEZ controllers. For a FlowEZ of 1000 mbar the maximum working pressure is 1100 mbar.
- FlowEZ pressure controller. The pressure controller can regulate the air inlet to the liquid reservoir to displace it.
- Link/Power supply module. The link module (linked to the FlowEZ pressure controller) allows the connection to a PC for software control.
- CAPs. Caps for the hermetic closure of reservoirs.
- Linking a flow sensor to the pressure controller allows direct control overflow regardless of pressure, which can vary as the reservoir empties. The flow sensor will be previous to the microfluidic device.

Figure 3. Schematic of the experiment set up of GOC model with BDF device and Fluigent® microfluidic system for the intestinal absorption studies.
3. Cell types
For the development of an intestinal monolayer, researchers consider two specific cell types:
First, researchers use the Caco-2 cell line (ATCC® HTB-37TM, USA). This cell line has been a standard in in vitro intestinal models since the 1990s. They culture these cells in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum (FBS). Additionally, the medium includes antibiotics, such as penicillin (100 U/mL) and streptomycin (30 µg/mL), as well as 1% non-essential amino acids (NEAA). Researchers then incubate the cells at 37 °C in a humidified atmosphere containing 5% CO₂.
In addition, researchers use the HT-29 cell line (ATCC® HTB-38TM, USA). This cell line is modified to a mucus-secreting cell type with methotrexate (MTX). They culture these cells in McCoy’s 5a Medium Modified, supplemented with 10% FBS and antibiotics. The antibiotics include penicillin (100 U/mL) and streptomycin (30 µg/mL). Afterward, they place the cells in a CO₂ incubator set at 37 °C, maintaining a humidified 5% CO₂ atmosphere.
By maintaining these two cell types under precise conditions, researchers ensure robust and reproducible intestinal monolayer models for further study.
4. Static intestinal model (control system)
Traditional culture inserts (0.4 µm porous size) adapted for 6-well cell culture plates are used as the control system.
Methods
1. Dynamic gastrointestinal digestion
The in vitro Dynamic Digester, developed by AINIA, simulates human gastrointestinal digestion. This computer-controlled system consists of interconnected compartments that replicate both gastric and intestinal digestions. The procedure occurs at 37 °C, in darkness, and under anaerobic conditions to closely mimic physiological conditions.
During the oral stage, the system simulates size reduction (chewing) and enzymatic digestion of the sample. To achieve this, the system disperses the sample at 37 °C in a simulated salivary solution containing α-amylase, maintaining a pH between 6.5 and 6.7. The mixture remains under agitation for 2 minutes at the same pH. For the gastric stage, the system introduces a solution containing stomach residuals, preheated to 37 °C (oral digesta). Next, it simulates gastric secretion with pepsin while controlling the pH curve by adding 1M HCl.
To simulate the transit from the stomach to the intestine, the system applies the Elashoff equation [3]. The subsequent gastric emptying mixes with intestinal media. Then, the system gradually adds a simulated intestinal solution containing pancreatin, electrolyte solution, and bile salts, maintaining a pH between 6 and 6.5. This creates the intestinal digestion media. The system also simulates intestinal emptying based on human in vivo data using the Elashoff equation.
So far, the total transit time amounts to 6 hours. AINIA has widely used this methodology to evaluate the effects of bioactive compounds in food supplements or ingredients [4-7].
The intestinal empties are accumulated generating the digestion blank.
2. Be-Doubleflow cell culture
Before seeding, prewarm the BDF device in the incubator overnight to minimise the formation of air bubbles.
- Fill the top channel of the device with 100 µL of 0.1 mg/mL of collagen or 0.1% gelatin (diluted in PBS) and incubate at 37°C for 30 minutes. Wash the channel by smoothly adding 100 µL of PBS into the inlet and removing it at the outlet well with the pipette. Repeat the washing step three times.
- Aspire gently the dilution buffer completely before seeding.
- Pipette through the pinhole of the apical channel 50 µL of culture medium with 106 Caco-2/HT-29/MTX (9:1) cells resuspended. Cover the inlets and incubate the cells for 5-6 h).
- After cell attachment, add 300 µL of culture medium to the medium reservoirs. Add PBS/water to the evaporation reservoirs, cover and keep the device in the incubator in the rocker. Every 2-3 days the media is renewed up to day 7.
3. Microfluidic set-up
Before setting the flow up:
- Sterilize and prewarm the tubes and fluidic elements overnight at 37 °C.
- Set the system in a laminar flow cabinet.
- The channels and inlet/outlet wells should never be depleted of culture medium.
- Both inlets and outlets are designed to be able to use connectors (1/4’’ – 28).
Important when working with cells:
To ensure sterile conditions while working with cells, begin by assembling the circuit under a laminar flow biosafety cabinet. First, autoclave the CAPs, pneumatic tubes, threaded connections, and ferrules you plan to use.
- Connect all circuit components except the microfluidic device.
- Establish a flow of ethanol (70%) for at least 15 min to sterilize the sensor and then wash with abundant sterile H2 Dry by passing air through at maximum pressure. The sensor CANNOT be autoclaved.
- Prime the tubes of the circuit of the top channel with digestive blank diluted in culture medium or the buffer and the bottom channel circuit with culture media or buffer until there are no air bubbles.
- Remove the culture medium from the chip reservoirs (not from inlet/outlet wells) and connect the tubes to the outlet/inlet using the threaded connections and ferrules.
- The ferrules must be manipulated with the help of sterile forceps.
- Ensure that the tube is perfectly fixed.
- Remove the displaced medium from the reservoirs.
- Once the system is closed, switch the flow on at 5.6 µL/min (shear stress 0.02 dyne/cm2) for 4 h. This flow rate is calculated taking into consideration the standard measures of the BDF channels. In case the size of the channel is customized, the flow must be recalculated.
- Observe the system under perfusion for a few minutes to check that there are no leaks.
Sensor Calibration
It is important to account for the sensor’s ethanol detection limit (0–70 µL/min for S FlowUnit). If this range is insufficient, change the liquid type to isopropanol (IPA) through the controller menu linked to the FlowUnit for better compatibility.
4. Dynamic intestinal absorption assay system
The “digested-like fluid” consists of digested blank diluted with HBSS or PBS without Ca2+/Mg2+ and is inserted through the inlet of the apical reservoir. The “blood-like fluid” is the buffer used in the dilution and inserted through the inlet of the basal reservoir (Figure 4). The outlet of the apical reservoir collects the not absorbed digested-like fluid while the basal reservoir collects the blood-like fluid with absorbed content.

Figure 4. Inlet and outlet connections of Gut-on-a-Chip model with Fluigent® microfluidic system for simulating intestinal absorption.
5. Static intestinal system
- Seed into an insert culture system Caco-2 and HT-29/MTX cells in 9:1 ration as seeding in Be-Doubleflow device.
- Use HBSS or PBS without Ca2+/Mg2+ as buffer for the digestion blank, same to the dynamic system.
- Incubate the solution with the cells for 4h before performing the cell viability assays.
6. Viability assay
To evaluate cell viability and proliferation during culture, perform assays such as the alamarBlue (DAL1025, ThermoFisher Scientific, USA) cell viability test. In this assay, resazurin, the active ingredient in alamarBlue, penetrates the cell membrane and is reduced to resorufin by live cells. This reaction produces a pink fluorescence that can be easily detected. Using an absorbance/fluorescence-based microplate reader, assess the quantitative number of cells and determine cell viability.
- At the chosen timepoint, remove the device from the incubator and place it in the cell culture hood.
- Remove the medium from the inlet and outlet reservoirs without emptying the cell channel.
- Wash the channel with PBS smoothly adding 100 µL of PBS into the inlet and removing it at the outlet well with the pipette. Repeat the washing step three times.
- Place 100 µL of the alamarBlue reagent in the inlet and wait for it to reach the outlet. If needed, slowly aspirate the liquid through the pinhole of the outlet.
- Place the device in the incubator for 1 h at 37 °C.
- Place the device in a plate reader or fluorescence spectrophotometer with 530/590 nm (excitation/emission) filter settings.
- To calculate the cell viability relative to the values obtained at 7 days, use the following equation:
7. Microscopy monitoring
The monolayer formation can be tracked through a phase contrast microscopy on specific timepoints (4 hours after incubation with digestion blank for the results presented in FIgure 7).
8. Total protein quantification
At the desired timepoint—after 4 hours of incubation with digestion blank in the results presented—extract the cells from the device for downstream analysis of parameters such as protein and RNA quantification. To perform cell extraction, refer to the detailed protocol in our technical note “Cell extraction in a Beonchip chip”.
For the presented results, begin by isolating the total protein content using the “CelLytic™ MT Cell Lysis Reagent” buffer (Sigma-Aldrich). Next, quantify the extracted protein using the Bradford protein assay kit (Bio-Rad). Measure the absorbance, and then extrapolate the results against a standard curve prepared with a known protein concentration (2000–125 µg/mL) to calculate the protein concentration accurately.
9. Total RNA quantification and evaluation
After cell extraction the RNA was isolated and purified using equipment’s such as Maxwell® RSC Instruments (Promega). The quantity and quality of the RNA isolated can be measured by Nanodrop, using A260/280 and A260/230 parameters as quality assessment. Values must be ≥1.8 to be considered free of contamination by DNA, proteins or aromatic compounds.
Results obtained using the guide protocol
Cell viability and morphology after digestion blank treatment
For the development of in vitro gastrointestinal digestive models, it is important to start the assays with controls such as the digestive blank (digestive fluids and enzymes without food/supplements). The composition of the digestive blank itself can damage the in vitro culture since the digestive enzymes present are active and therefore, can detach the cells and the bile salts at certain concentrations can be toxic for the cells. To avoid excess of cell toxicity, the digestive blank can be diluted but the specific dilution buffer and dilution factor depend on the cell culture. Previous cell viability assays are then recommended to determine the dilution factors that has to be applied to ensure biocompatibility of the digestive blank without food/supplements added [2].
a) Transwell (static)
First, the cell viability after 4 h exposure to the digestion blank was evaluated in the static model. Samples included the digestion blank diluted with HBSS 1:5; 1:10 or 1:20; digestion blank diluted with PBS 1:5; 1:10 or 1:20 and cytotoxic positive control composed of DMSO 20%. Results showed that to keep high cell viability, digestion blank needs to be diluted at 1:10 with HBSS while in PBS the dilution needed is 1:20 (Figure 5).

Figure 5. Cell viability of serial dilutions of digestion blank in HBSS and PBS in the transwell (static). C+ = cytotoxic positive control (DMSO 20%).
b) Be-DoubleFlow compared to transwell (Control)
Cell viability
The cell viability was assessed in a BDF device cultured for 7 days and exposed to the digestion blank for 4 h in dynamic conditions (Figure 6). The digestion blank was diluted with HBSS or PBS in a 1:5 or 1:10 dilution and compared with static model.
Results showed higher cell viability in the dynamic model in all conditions when compared with static model. Additionally, the dilution necessary to keep high cell viability in HBSS was 1:5 in the dynamic model, while static model needed a dilution of 1:10. In PBS, the dilution necessary in the dynamic model was 1:10, while in static conditions it needed to be 1:20 (as shown above in Figure 5).
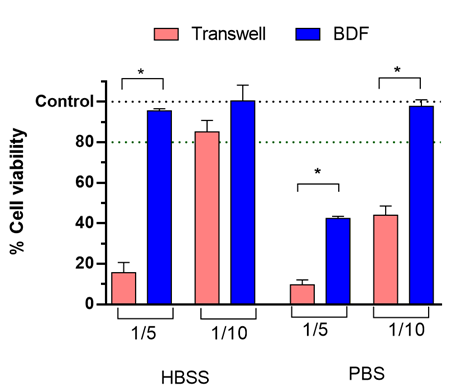
Figure 6. Cell viability of digestion blank under same conditions in static model vs dynamic model (GOC) in HBSS or PBS buffers. *p<0.05.
More concentrated digestion blanks equal better limits of quantification of the possible compounds of interest for uptake studies, proving that the GOC model is more advantageous as absorption models than the static control. The reason behind this improved result in the GOC system might be associated with the higher expression of mucins this model presented when compared with static culture since mucins protect the cellular epithelium (results presented in the technical note “Be-Doubleflow App. notes: Gut-on-chip 1”.
Cell morphology
These results were further supported by the evaluation of the cell morphology through contrast microscopy. Figure 7 demonstrates the results obtained at 1:5 and 1:10 dilution with PBS in both dynamic and static models. In static model, both dilutions provoked gaps in the cell monolayer, especially the 1:5 dilution. In the dynamic model a confluent monolayer is visible in both conditions. Additionally to the cell viability results, it can be deduced that even though the monolayer is maintained in the dynamic model, the integrity of the monolayer can be compromised in the 1:5 dilution. It can also be seen that the clear hexagonal morphology of the cells with thigh junction between cells is slightly lost after exposure with 1:5 diluted digestion blank in the dynamic model.

Figure 7: Phase contrast images of the cell (Caco-2: HT-29/MTX) monolayer on the control system (Transwell) and Be-Doubleflow at day 4 and day 7 of culture.
Moreover, we assessed the viability of the culture before and after the 4 h incubation with the digestion blank, comparing the static with the dynamic system applying same conditions in terms of buffer and dilution. Results showed no significant differences between timepoints and conditions, which validates the non-toxicity of the treatment (Figure 8).

Figure 8. Cell viability of the Transwell and Be-Doubleflow device before (T=0) and after (T=4h) the 4h of intestinal absorption simulation.
Total RNA and protein content
RNA and protein content and quality of the sample is important when using both for further determinations, including omics assays (eg. transcriptomic or proteomic analysis). Comparing insert culture and Be-Doubleflow device, results showed similar RNA and protein content on both systems, with the protein levels being slightly higher (p<0.1) on the De-Doubleflow device. The quality of RNA was also similar in both systems. These results corroborate the suitability of GOC systems to be used as standard intestinal models.

Figure 9. Total RNA and protein quantification and quality assessment. (A) RNA and protein levels on cells cultures in control (Transwell) and Be-Doubleflow device (GUTOnChip) after 4 h of incubation with digestive blank. (B) A260/280 and A26/230 values obtained through Nanodrop analysis as a marker for RNA quality.
Conclusions
This study concludes that the Be-Doubleflow device is a suitable platform for a gut model. When connected with Fluigent® microfluidic pumps, it becomes an effective tool for dynamic intestinal absorption in vitro, offering advantages over the static control model. The dynamic model developed in this study enabled the use of a less diluted sample of the digested material. This approach not only reduces sample manipulation but also brings the process closer to the in vivo situation. Additionally, using a less diluted sample enhances the quantification analysis of the components present. Moreover, isolating the total protein and RNA content from the Be-Doubleflow devices proved straightforward, and the quantity and quality of the samples were similar to, or slightly higher than, those obtained from the control system.
Download the full Application note
References
- Volpe, D. A. Advances in cell-based permeability assays to screen drugs for intestinal absorption. Expert Opin. Drug Discov. 15, 539–549 (2020).
- Kondrashina, A. et al. Coupling in vitro food digestion with in vitro epithelial absorption; recommendations for biocompatibility. Crit. Rev. Food Sci. Nutr. 64, 9618–9636 (2024).
- Blanquet, S. et al. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm. Res. 21, 585–591 (2004).
- Nieto, J., Soriano-Romaní, L., Tomás-Cobos, L., Sharma, L. & Budde, T. Improved in vitro bioavailability of a newly developed functionalized calcium carbonate salt as a food ingredient and its comparison with available commercial calcium salts. Food Chem. 348, (2021).
- Bryszewska, M. A. et al. In vitro bioaccessibility and bioavailability of iron from breads fortified with microencapsulated iron. LWT 99, 431–437 (2019).
- Soriano-Romaní, L., Nieto, J. A. & García-Benlloch, S. Immunomodulatory role of edible bone collagen peptides on macrophage and lymphocyte cell cultures. Food Agric. Immunol. 33, 546–562 (2022).
- Soriano-Romaní, L., Nieto, J. A., Tomás-Cobos, L. & Díez-Sánchez, E. Modulatory activity of a bovine hydrolyzed collagen-hydroxyapatite food complex on human primary osteoblasts after simulating its gastrointestinal digestion and absorption. Nutr. Hosp. 39, 644–651 (2022).
Related products from Beonchip/Fluigent:
- Be-Doubleflow
- Microfluidic flow control system connection kit
- FlowEz
- LineUP link
- FlowEZ supply kit
- Flow Unit S
- P-CAPs
- Pressure source FLPG+

