Summary
Reproducing the vascular microenvironment in vitro requires not only dynamic culture systems, such as microfluidic organ-on-chip devices, but also the appropriate extracellular matrix (ECM) coating. Laminins, key components of the basal lamina, provide specific biochemical cues that regulate endothelial cell adhesion, morphology, and function. In this study, Laminin-521 (LN521), Laminin-411 (LN411), and gelatin were compared as coatings for huVEC culture under static and shear stress conditions. Results showed that LN521 supported superior adhesion, spreading, and stability, both in static and dynamic conditions, making it the most suitable substrate for endothelial microfluidic models. These findings highlight the importance of ECM selection for advancing physiologically relevant in vitro vascular systems with applications in drug testing, tissue engineering, and the reduction of animal use.
Introduction
Reproducing the physiological microenvironment of endothelial cells in vitro is essential but challenging. While static cultures on coated substrates provide valuable insights, they do not capture the hemodynamic forces that endothelial cells experience in vivo, such as shear stress, pulsatile flow, or pressure fluctuations (1,2). In contrast, dynamic culture systems, especially those based on microfluidic organ-on-chip technology, allow researchers to simulate these biomechanical cues, providing a more physiologically relevant model of the vascular niche (3).
However, setting appropriate culture conditions is as important as the choice of system. Several factors determine the success and reproducibility of endothelial models: cell seeding density, culture duration, type and magnitude of flow, and substrate composition (4). Among these, the extracellular matrix (ECM) coating is particularly critical, as it provides both structural support and biochemical signals that regulate adhesion, survival, and differentiation (5).
How to choose the best ECM coating
The choice of ECM coating should consider the cell type as well as the tissue or organ of origin. Laminins, a large family of multidomain glycoproteins, are key components of the basal lamina that supports most tissues. Each organ and vascular bed are characterized by a distinct combination of laminin isoforms, which provide specific cues for cell attachment, polarity, and signaling. Importantly, only intact full-length laminins present the full repertoire of binding domains for cellular receptors and growth factors, thereby enabling co-signaling mechanisms that recreate an authentic microenvironment (6,7). The use of animal origin-free laminins also reduces variability and enhances translational relevance compared to undefined coatings such as gelatin.
In this study, we systematically tested different ECM coatings to identify the most suitable conditions for endothelial cell culture within a microfluidic device exposed to shear stress. Specifically, we compared Laminin-521 (LN521) and Laminin-411 (LN411) with gelatin, which is commonly recommended by commercial suppliers as a standard coating.
Laminin-521 is abundant in vascular basement membranes and niches, where it promotes stable adhesion, spreading, and maturation of endothelial monolayers (8). In contrast, Laminin-411, found predominantly in vascular and lymphatic basement membranes, plays a key role in cell migration, sprouting, and angiogenic processes (9).
Methods
Coating selection in static culture
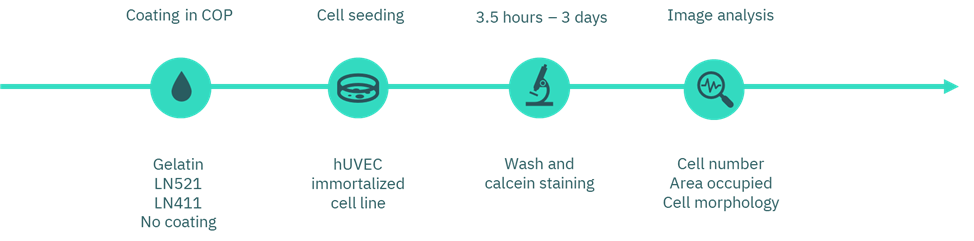
Figure 1. Workflow diagram of coating selection.
First, we prepared the coatings using gelatin (Inscreenex, INS-SU-1015-50), Laminin-521 (LN521, BioLamina, LN521-02), Laminin-411 (LN411, BioLamina, LN411-02), and uncoated wells as controls. A commercial cyclic olefin polymer (COP)-based platform with 96-well plate format wells was used.
We applied the gelatin coating at manufacturer’s recommended concentration of 0.5%. In parallel, we prepared the laminin coatings at a concentration of 10 µg/mL. All coating solutions incubated overnight at 4 °C. The next day, we carefully removed the solutions without washing prior to cell seeding.
Next, we seeded human umbilical vein endothelial cells (huVEC; CI-huVEC, INS-CI-1002) in culture medium (INS-ME-1011) at a density of 2000 cells per well. After 3.5 h and again at 3 days of incubation, we gently washed the wells with PBS and incubated them with calcein to fluorescently label viable cells. Finally, we assessed cell morphology and number by imaging and performed quantitative analysis using Fiji (ImageJ).
Culture under flow
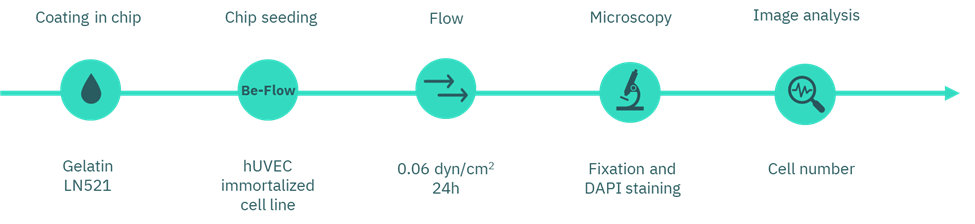
Figure 2. Workflow diagram of flow assay.
We chose the Be-Flow microfluidic device to perform the experiments, since it is optimized for vascular models and blood vessel simulation. The device is compatible with any cell types and ECM coatings, and its optical properties allow high-resolution imaging with high-magnification and immersion objectives.
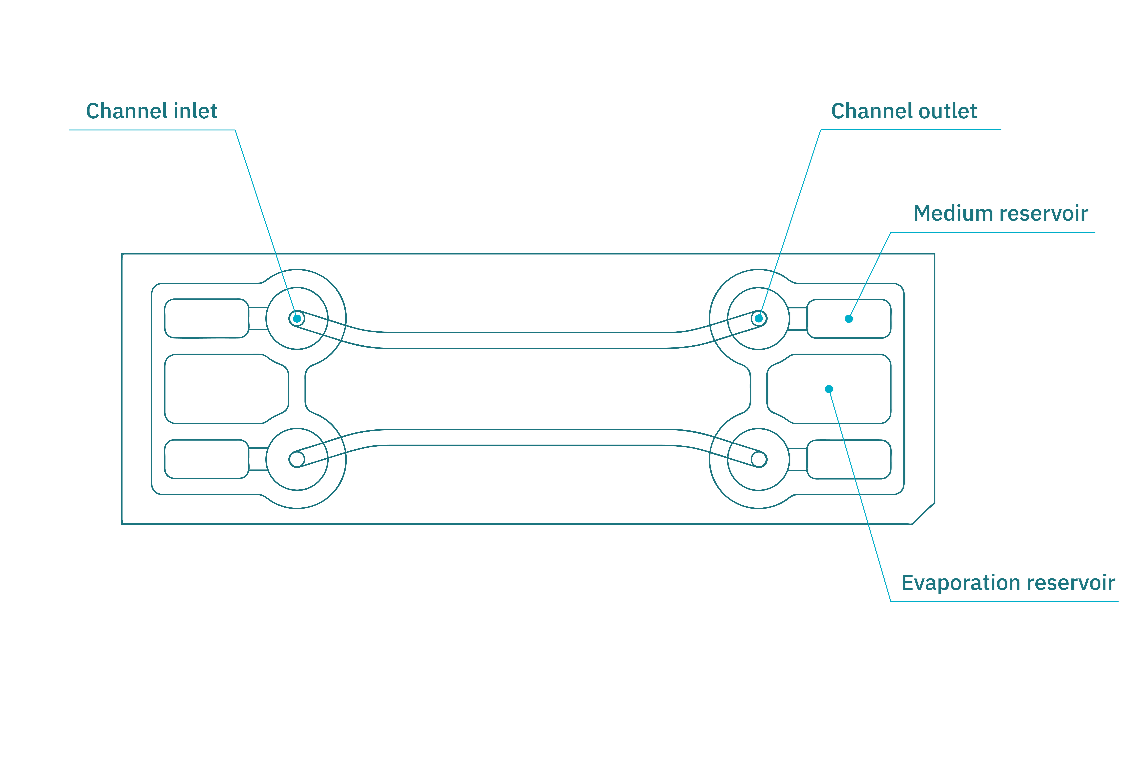
Figure 3. Schematic illustration of the Be-Flow organ-on-chip platform. Each chip contains two independent channels, enabling cell culture under static or perfusion conditions. Labeled parts correspond to medium reservoirs, inlet/outlet and observation area of the channel.
In its standard configuration, the chip contains two independent rectangular channels (270 µm in height, 1.5 mm in width). See figure 3.
Prior to use, we pre-warmed the devices overnight in the incubator . Each chip hosted two coating conditions, one channel with gelatin and the other with Laminin-521 (concentrations described in the Coating selection section).
For coating, we injected 30 µL of solution into each channel and incubated for 2 h at 37 °C. Afterwards, we carefully aspirated the coating solutions without washing.
Next, we seeded 100,000 huVEC in 30 µL per channel. After 2 h of attachment, we added culture medium (400 µL/channel; 200 µL per medium reservoir) to fill the reservoirs. Finally, we incubated the devices overnight under rocking conditions to promote homogeneous medium exchange. Here you can visualize a how-to-do video.
Perfusion culture
On the following day, we connected the to the perfusion system using the Flexible Tubing Kit for Syringe Flow (Figure 4). A 20 mL syringe (not included in the kit) was used to deliver medium flow. The syringe pump was programmed to apply a shear stress of 0.6 dyn/cm² for 24 h.
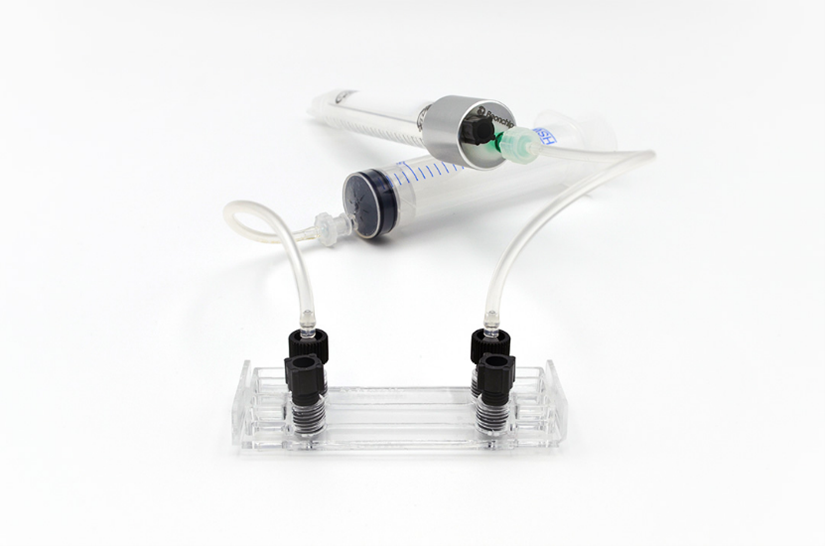
Figure 4. Be-Flow channel connected to the perfusion setup, including flexible tubing, a syringe, and a multi-system CAP.
Fixation and staining
At the end of the experiment, we fixed the cells with 4% paraformaldehyde (PFA) following the fixation protocol. Nuclei were stained with DAPI, and cell number per field of view was quantified along the channel surface.
| Product | Provider | Link | |
| Coatings | Gelatin | Inscreenex | Gelatin |
| Biolaminin® 521 (LN521) | BioLamina | Laminin-521 | |
| Biolaminin® 411 (LN411) | BioLamina | Laminin-411 | |
| Cells | Human Umbilical Vein Endothelial Cells (huVECs; CI-huVEC) | Inscreenex | huVEC |
| Medium Kit for CI-huVEC Endothelial Cells | Inscreenex | huVEC Medium | |
| Culture platform | Be-Flow Microfluidic Device | Beonchip | Be-Flow |
| Flow accesories | Flexible Tubing Kit for Syringe Flow | Beonchip | Perfusion kit |
Table 1. Summary of references.
Results
Short- and mid-term endothelial adhesion
The first step in this work was to determine which ECM protein provided the most suitable substrate for short- and mid-term endothelial adhesion. To this end, we quantified the number of cells adhering to culture surfaces coated with different ECM proteins at 3.5 hours post-seeding. At this early stage, cells under favorable anchorage conditions efficiently initiate their first contacts with the substrate.
The results showed that the number of adherent cells was similar for gelatin, Laminin-411 (LN411), and the uncoated control (Figure 5B), ranging between 1400–1600 cells. In contrast, surfaces coated with Laminin-521 (LN521) supported markedly higher adhesion, exceeding 2000 cells. These differences became even more pronounced after 3 days of culture (Figure 6B), with approximately 1500 cells remaining on LN521, compared with <900 on gelatin, <700 on L411, and fewer than 300 counted cells in the control condition.
Cell morphology
In addition to adhesion, cell morphology (Figure 5C and 6C) was assessed through the parameter of circularity (circularity = 4π × [area/perimeter²]). A circularity value of 1.0 corresponds to a perfect circle, while values approaching 0.0 reflect elongated or polygonal shapes. Following trypsinization and detachment from flasks, cells in suspension appear spherical with a circularity close to 1.0. Once seeded, cells adhere, spread, and flatten on the substrate, adopting elongated or polygonal morphologies that reduce their circularity depending on cell type and substrate interaction.
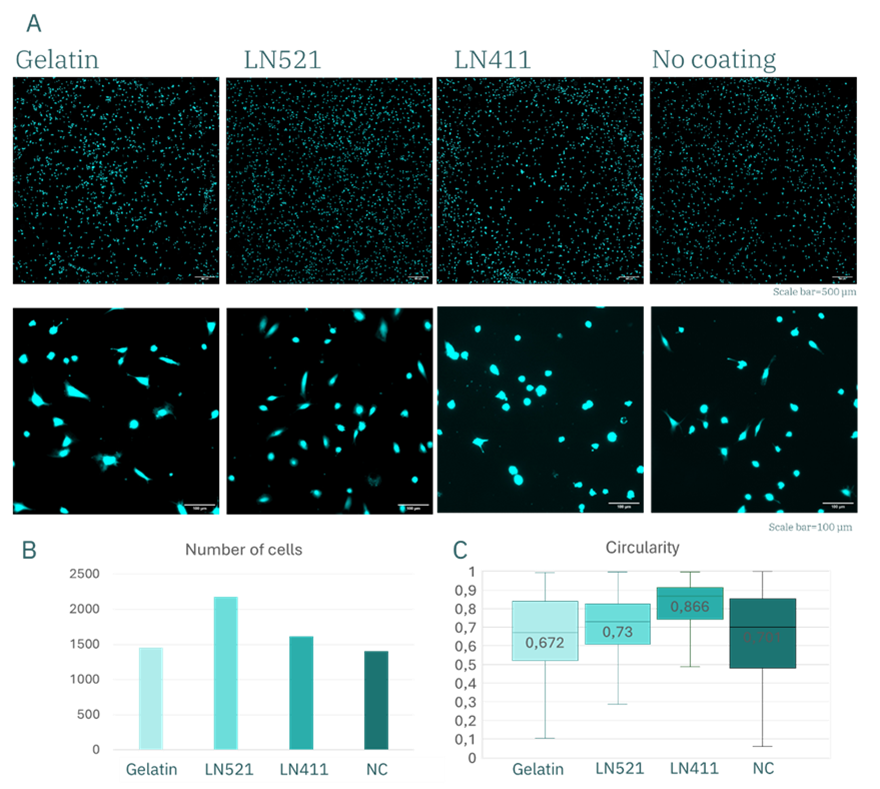
Figure 5. huVECs after 3.5 h of culture. (A) Fluorescence images of calcein-stained huVECs and representative graphs showing quantitative analysis of cell number (B) and morphology (C).
After 3.5 hours of culture, circularity values were comparable (~0.7) for gelatin, LN521, and the control, with slightly higher values observed for LN411. By day 3, however, these differences were accentuated: circularity decreased to ~0.4 for gelatin and LN521, while it remained at ~0.7 for LN411. This indicates that cells cultured on gelatin and LN521 substrates acquired a more elongated, spindle-like morphology consistent with healthy adhesion and spreading, whereas cells on LN411 retained a rounded shape, indicative of poor adhesion and compromised cell health. Combined with the marked preservation in cell number, these findings demonstrate that LN521 is the optimal coating for huVEC culture under these conditions.
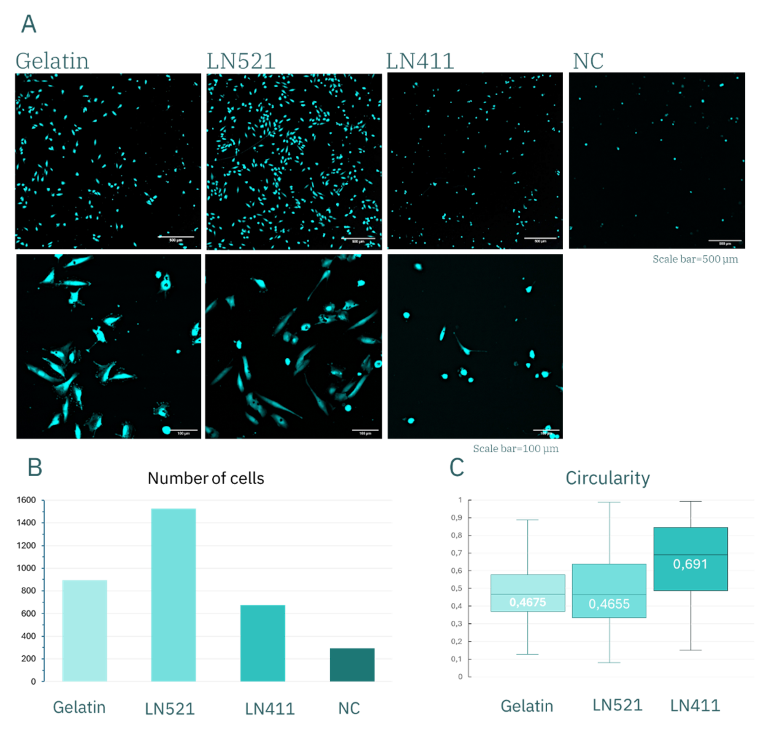
Figure 6. huVEC after 3 days of culture. (A) Fluorescence images of calcein-stained huVEC and representative graphs showing quantitative analysis of cell number (B) and morphology (C).
Gelatin versus LN521 under shear stress
Based on these results, gelatin and LN521 were selected as coating conditions for flow experiments in the Be-Flow microfluidic device. As shown in Figure 7, after 24 hours of perfusion at 0.06 dyn/cm², endothelial monolayers were disrupted in gelatin-coated channels, whereas LN521 maintained an intact, confluent monolayer. Under shear stress, cell detachment was approximately two-fold higher in gelatin compared with LN521.
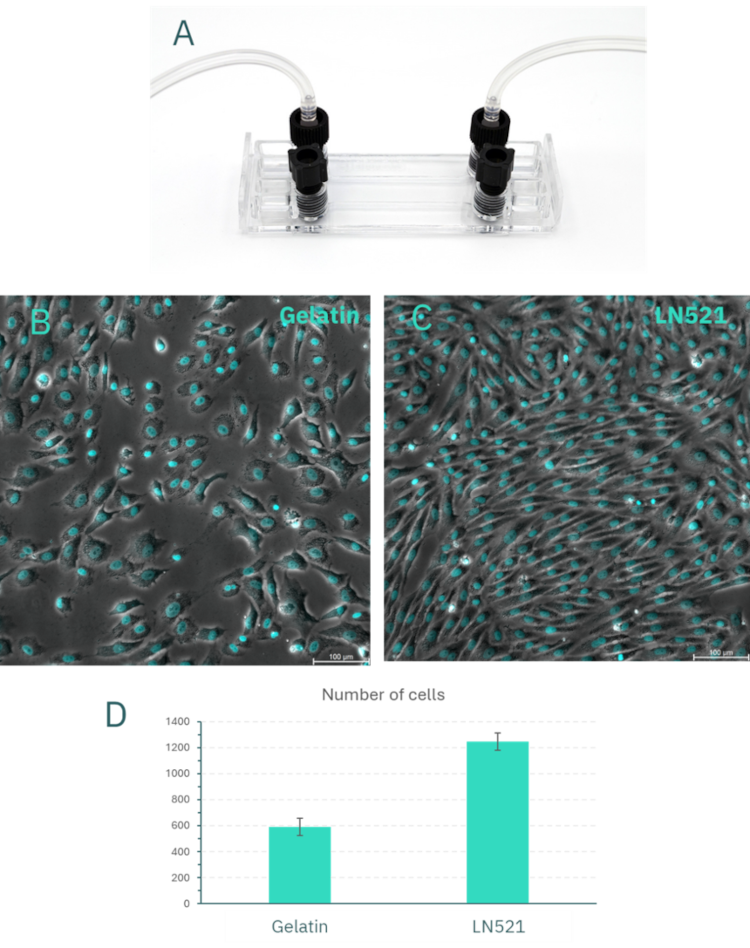
Figure 7. Flow assays of huVEC in the Be-Flow device. (A) Image of the Be-Flow microfluidic device with threaded connections to flexible tubing. Representative fluorescence images of endothelial cells after perfusion with culture medium at 0.06 dyn/cm² on gelatin coating (B) and Laminin-521 coating (C). (D) Quantification of the number of cells per field for both coating conditions.
Conclusions
Taken together, these findings demonstrate that Laminin-521 (LN521) not only provides optimal adhesion and morphology for huVEC culture under static conditions but also ensures superior stability and resistance under microfluidic shear stress.
By integrating microfluidic dynamic culture with carefully selected laminin isoforms, endothelial in vitro models can more accurately reproduce the complexity of vascular biology, advancing both basic research and translational applications such as drug testing, vascular tissue engineering, and reduction of animal experimentation.
For further information, please contact us at info@beonchip.com
Our team will provide the necessary support and guidance.
References
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Microengineered physiological biomimicry: Organs-on-chips. Lab Chip. 2011;11(22):3341–7.
- Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–72.
- Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci U S A. 2011;108(37):15342–7.
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100(2):158–73.
- Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–86.
- Yousif LF, Di Russo J, Sorokin L. Laminins in vascular development and disease. Matrix Biol. 2013;32(5):303–11.
- Domogatskaya A, Rodin S, Tryggvason K. Functional diversity of laminins. Annu Rev Cell Dev Biol. 2012;28:523–53
- Rodin S, Domogatskaya A, Ström S, Hansson EM, Chien KR, Inzunza J, et al. Clonal culturing of human embryonic stem cells on laminin-521 supports stable self-renewal and differentiation. Nat Commun. 2014;5:3195
- Hall A, Khan S, Naranjo JD, Hassell T. Laminin-411 enhances endothelial differentiation from human induced pluripotent stem cells. Front Cell Dev Biol. 2022;10:874003.

