Summary
This paper describes the use of our Be-Gradient Barrier-Free device to simulate the ischemic conditions present in solid tumors, enabling the study of volatile organic compounds (VOCs) as biomarkers for tumor progression . This approach marks a notable advancement in exploring VOCs in early tumor detection, with promising implications for improving patient outcomes – especially in the case of hard-to-reach and fast-growing tumors like glioblastoma.
Read the full article here.
Introduction
Liquid biopsy
Liquid biopsy offers a minimally invasive method to detect, monitor and characterize cancers, providing advantages over traditional tissue biopsies. It enables real-time tracking and early tumor detection through straightforward diagnostics[1]. Liquid biopsies are particularly valuable for cancers like glioblastoma, where tissue biopsies are often inconclusive or unfeasible due to surgical risks[2-3]. Unlike tissue biopsies, liquid biopsies capture a tumor’s evolving heterogeneity by allowing repeated sampling over time. Thus, this approach enhances cancer monitoring by detecting tumor progression, evaluating treatment response, and identifying recurrence more effectively.
In addition, volatile organic compounds (VOCs) present in liquid biopsies are organic molecules with boiling points below 250°C, produced through cellular metabolism. Once formed, these molecules enter the bloodstream and can be detected in blood, breath, urine, or skin emissions[4]. Importantly, VOCs reflect biological activities like cell death, inflammation, or oxidative stress by indicating changes in metabolite levels[5]. Therefore, in vitro VOC detection effectively isolates tumor-specific gases, minimizing external pollution effects and reducing misleading results. This makes volatile organic compound detection, an optimal biomarker for the analysis of the tumor state.
Be-Gradient for VOC detection
The main challenge of in vitro VOC detection is replicating the in vivo conditions that allow for VOC secretion [6]. Most microfluidic platforms are made from polydimethylsiloxane (PDMS), with high oxygen and water vapor permeability. In contrast, Beonchip’s devices use cyclic olefin polymer (COP), which offers extremely low permeability. This unique property ensures better control of gas exchange within the microchannel.
Researchers can precisely manage oxygen and water vapor levels by regulating gas concentration in the culture medium and controlling its flow. Importantly, oxygen does not diffuse through the chip’s surface, preventing unintended exchanges. Instead, cells cultured on Beonchip devices receive oxygenated medium through the tumor’s outermost region. This setup effectively mimics the oxygen and gas gradients found in solid tumors in vivo.
Consequently, Beonchip devices provide an advanced system for replicating in vivo-like conditions for VOC detection. Their design overcomes the limitations of traditional microfluidic platforms, making them a valuable tool for tumor research.
By using the Be-Gradient Barrier-Free device, researchers created a system that stops atmosphere VOCs from mixing with the ones generated by the cell metabolism, and simultaneously, they ensured that the cells are alive through the perfusion of a continuous supply of oxygenated medium.
Experimental Setup
Researchers used the Be-Gradient Barrier-Free device to recreate glioblastoma or colorectal cancer models under controlled conditions. They resuspended the cells in high-density collagen type I and introduced them into the device’s central chamber. After allowing the hydrogel to polymerize, they added the cell culture medium to the lateral channels to support cell growth. A low-density cell model was also created for comparison studies.
Over time, the high cell concentration within the central chamber formed a necrotic core, mimicking the tumor’s in vivo structure. To enable VOC collection, researchers connected the device’s inlets to a syringe pump and attached gas-impermeable tubes to the outlets. This setup ensures a closed system for precise VOC sampling.
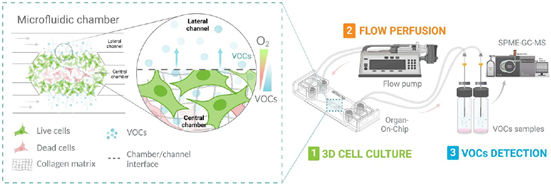
Figure 1: Experimental Setup. 1. 3D cell culture using the Be-Gradient Barrier-Free to recreate the hypoxic tumour microenvironment. 2) Connection of the 3D model to a perfusion system (syringe pump) and collection of cell culture medium after contact with cells. 3. VOC sample analysis using SPME-GC-MS equipment.[7]
Next, researchers collected the medium in contact with the cells over 24 hours, at two distinct time points. Finally, they analyzed the collected samples using headspace solid-phase microextraction gas chromatography-mass spectrometry (HS-SPME-GS-MS). This approach provided detailed insights into the VOCs released by glioblastoma or colorectal cancer cells, advancing tumor model research.
Main findings
The Model Simulates Glioblastoma’s In Vitro Tumor Microenvironment (TME)
The Be-Gradient Barrier-Free device and specific cell-seeding conditions enabled the generation of a hypoxic core within seven days. This core, a hallmark of solid tumors, validated the model’s ability to recreate glioblastoma’s TME accurately.
Distinct VOC Profiles Differentiate Glioblastoma and Colorectal Cancer Tumors
Tumor conditions vary based on their type. This model aimed to study the gas profiles of tumors created under the same hypoxia-inducing protocol but using different cell types. The volatile compound profiles for glioblastoma and colorectal cancer differed significantly. These results confirmed the model’s capacity to simulate unique TMEs for distinct tumors with the same protocol.
Tumors Exhibit Unique Metabolic Changes During Progression
Tumor models with necrotic cores (day 7 of culture) showed significantly different VOC levels compared to those without (day 1 of culture). Additionally, each tumor type exhibited a distinct VOC profile, underscoring the metabolic differences between tumors during their progression.
Hypoxic Microenvironment Drives Glioblastoma-Specific VOC Production
Researchers compared the impact of a hypoxic core on VOC profile to assess its importance for accurate in vitro modelling. In models without a hypoxic core, VOC profiles remained consistent over time. Conversely, hypoxia-inducing models displayed distinct VOC profiles as the culture progressed, demonstrating the hypoxic core’s critical role in mimicking in vivo tumors.
In summary, the 3D model established with the Be-Gradient Barrier-Free allowed not only the recreation of a necrotic core within a tumor model but also the study of the VOCs released by the tumor cells, which other in vitro models fail to do.
You can find more information regarding the use of Volatile organic compound detection as biomarker for tumor state adn progression in the full article.
More information about the Be-Gradient Barrier-Free device and how to use it is available on our website.
References
- R. Di Bonaventura et al., “Reassessing the role of brain tumor biopsy in the era of advanced surgical, molecular, and imaging techniques—a single-center experience with long-term follow-up,” J. Pers. Med., vol. 11, no. 9, p. 909, 2021.
- M. Riche, A. Amelot, M. Peyre, L. Capelle, A. Carpentier, and B. Mathon, “Complications after frame-based stereotactic brain biopsy: a systematic review,” Neurosurg. Rev., vol. 44, pp. 301–307, 2021.
- J. Müller Bark, A. Kulasinghe, B. Chua, B. W. Day, and C. Punyadeera, “Circulating biomarkers in patients with glioblastoma,” Br. J. Cancer, vol. 122, no. 3, pp. 295–305, 2020.
- I. Belluomo et al., “Selected ion flow tube mass spectrometry for targeted analysis of volatile organic compounds in human breath,” Nat. Protoc., vol. 16, no. 7, pp. 3419–3438, 2021.
- G. Lubes and M. Goodarzi, “GC–MS based metabolomics used for the identification of cancer volatile organic compounds as biomarkers,” J. Pharm. Biomed. Anal., vol. 147, pp. 313–322, 2018.
- Z. Jia, A. Patra, V. K. Kutty, and T. Venkatesan, “Critical review of volatile organic compound analysis in breath and in vitro cell culture for detection of lung cancer,” Metabolites, vol. 9, no. 3, p. 52, 2019.
- C. Bayona, M. Wrona, T. Randelovic, C. Nerín, J. Salafranca, and I. Ochoa Garrido, “Development of an organ-on-chip model for the detection of volatile organic compounds as potential biomarkers of tumour progression,” Biofabrication, 2024.

