In this note, we summarise the possible combination of two technologies, organoids and microfluidic devices, in what is also known as organoids-on-chip.
What is an organoid and why use it in research?
There has been an increasing shift towards the development of 3D cell culture models in attempts to create an increased complexity that can be compared with the in vivo better than 2D models. From the different 3D models, cell aggregates are particularly interesting in mimicking organs and tumours because of the close cell-cell interactions in multiple planes, self-organization and gene and protein expression they reflect [1].
Spheroids
Cell aggregates can be classified as either spheroids or organoids. Both arise from the compact assembly of cells into a 3D structure, but they differ in composition. Spheroids consist of cells derived from commercial cell lines, single-cell suspensions, or tissues. These cells organize themselves into a structure resembling solid tumors, including proliferative, quiescent, and hypoxic regions. This structural similarity translates into a comparable respons to chemotherapeutics, making spheroids highly advantageous for drug screening assays. As a result, they enable better prediction of drug outcomes and facilitate more efficient transitions to in vivo studies [2].
Moreover, spheroids can be composed of various cell types, forming multicellular spheroids that provide an even more representative model for studying complex cellular interactions.
Organoids
Organoids form from stem cells that differentiate into specific cell types and self-organize into structured 3D models. To support this self-organization, researchers commonly embed organoids in a matrix, which facilitates maintenance and stability [3]. Although multicellular spheroids and organoids may seem similar, key differences set them apart. While multicellular spheroids exhibit disorganized cell proliferation within a cluster, organoids develop into organized and polarized structures, often featuring lumens, as seen in gastrointestinal and lung organoids [4]. This polarization, along with the presence of lumens, enables organoids to support advances studies – from cell-cell interactions to drug behaviour – that 2D models cannot replicate.
To date, researchers have successfully generated more than 15 types of organoids to study organs such as the brain, liver, kidney, and intestine. These organoids have advanced fields like embryogenesis, organogenesis, pathogenesis, drug discovery, and personalized medicine. Furthermore, some organoids exhibit specific organ functions, such as neural activity in brain organoids or periodic contractions in cardiac organoids, further enhancing their utility in biomedical research [5,6].
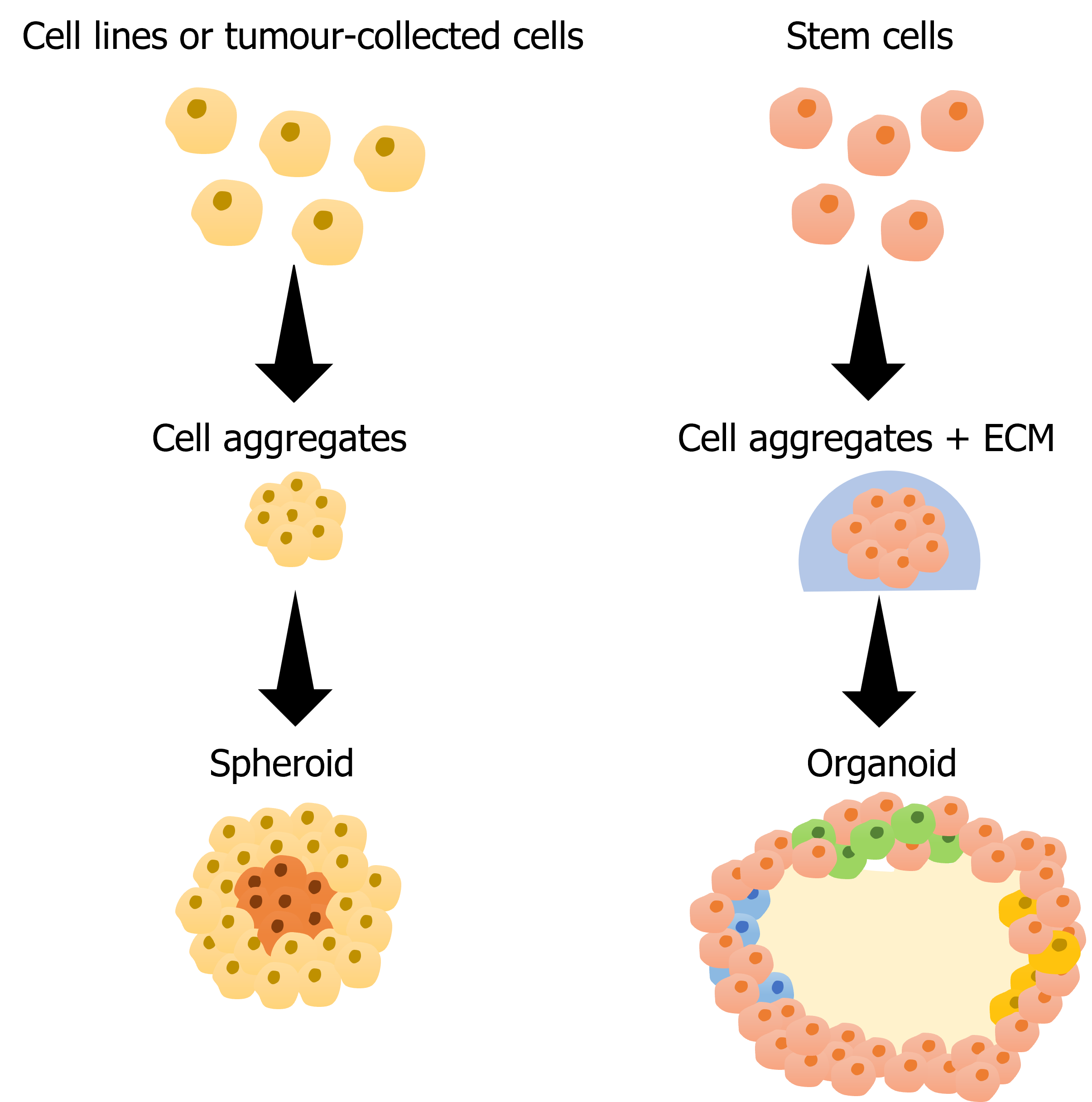
Figure 1 Scheme of spheroid and organoid production. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
In most diseases, researchers recognize that not all patients respond equally to existing treatments, which significantly impacts drug effectiveness. Spheroids and organoids, produced from patient-derived cells, can differentiate into healthy or diseased models that more accurately reflect the patient’s condition. This capability is highly advantageous for selecting the most efficient treatment for an individual patient, ultimately increasing survival rates and enabling more personalized medicine [7].
Disadvantages of cell aggregates
However, despite their promise, organoids still present certain disadvantages [8]. For example, most organoid protocols require several weeks for the models to mature sufficiently for assays. During this time, organoids grow in size, often reaching a few millimeters, which complicated medium diffusion and leads to the formation of hypoxic centers. While tumor spheroids can leverage hypoxic cores, as these are characteristic of human tumors, healthy organoids face a different challenge. Hypoxic centers do not reflect the conditions of healthy organs, limiting the accuracy of these organoid models in mimicking their intended targets.
Why combine organoids and microfluidic devices?
- Decrease of hypoxia conditions in the centre of the organoid
Microfluidic devices are compartmentalized platforms capable of introducing architecture and mechanical cues to cell culture. These models are particularly interesting to be coupled with organoid culture. One of the most interesting characteristics of organoids-on-a-chip is the exposure of the culture to continuous fluid flow, which increases the diffusion of cell media and decreases hypoxia on the organoid core [9]. The inclusion of a perfusion system also automates the system by decreasing the need for human manipulation of organoid culture, especially the need to frequently change the medium manually.
-
Introduction biomechanical and biochemical force stimuli
The use of flow in culture provides multiple benefits, including the prevention of hypoxic conditions and the introduction of shear forces and gradients. These forces actively influence biological processes by activating mechano-proteins and enhancing the vascularization of organoids [10]. Furthermore, mechanical forces play a crucial role in the development and maturation of organs. However, achieving biomechanical control over these forces in 2D or 3D models remains challenging. To address this issue, researchers are actively developing microfluidic devices designed to be mechanically active, enabling the application of these forces to organoid cultures [11,12].
During organogenesis, the organization of different tissue layers depends on exposure to specific stimuli at precise time points. In traditional 2D cultures, it is difficult to expose cells to varying stimuli, such as chemical and physical signals, within the same well. In contrast, microfluidic devices, with their distinct compartments and channels, can introduce physical stimuli to specific areas or generate chemical gradients. This capability offers greater control over organoid differentiation, promoting maturation and better mimicking native organs [13]. Additionally, the ability to create chemical gradients through microfluidic devices proves highly valuable in drug screening (Figure 3A) [14,15]. By combining channels and wells, these devices allow researchers to culture a high number of spheroids or organoids and expose them to varying drug concentrations in a single assay.

Figure 2 Summary of advantages of combining microfluidic devices with organoid culture.
-
Production of homogeneous replicates
Organoid production is often linked with the generation of heterogeneous replicas. This can be a disadvantage since different-sized organoids cannot be considered triplicates in experiments. To aid the production of organoids, microfluidic devices capable of generating droplets can be used (Figure 3 B). Using droplet microfluidics to produce organoids allows the generation of homogeneous replicas at a fast pace, permitting the high throughput production of these models [16,17].
Microfluidic devices can act not only as homogeneous organoid generators but also as a filter to separate organoids with different sizes and allow the collection of similar-size replicas (Figure 3 C) [18,19].
-
Use of smaller volumes and decreasing expenses
Stem cell differentiation protocols are usually associated with long-term cultures and expensive cell culture media. Organoid culture is also associated with these characteristics. The pairing of organoid culture and microfluidic platforms can decrease the expenses with media culture since microfluidic devices use a smaller volume of liquid to nourish the cultured cells.
-
High-throughput production and maintenance
For drug screening assays, the ability to perform high throughput culture and analysis is very interesting, especially from an industry point of view. As mentioned above, droplet microfluidic platforms can produce homogeneous and high-quantity organoids. Besides this massive production, microfluidic devices are also able to serve as a culture and maintenance platform for a high number of organoids, such as a microwell array (Figure 3 D) [20,21]. Depending on the design, this platform can act as only maintenance where all organoids are exposed to the same cultured media or organoids can be seeded in different compartments or channels and exposed to different media or different concentrations of media/substances.
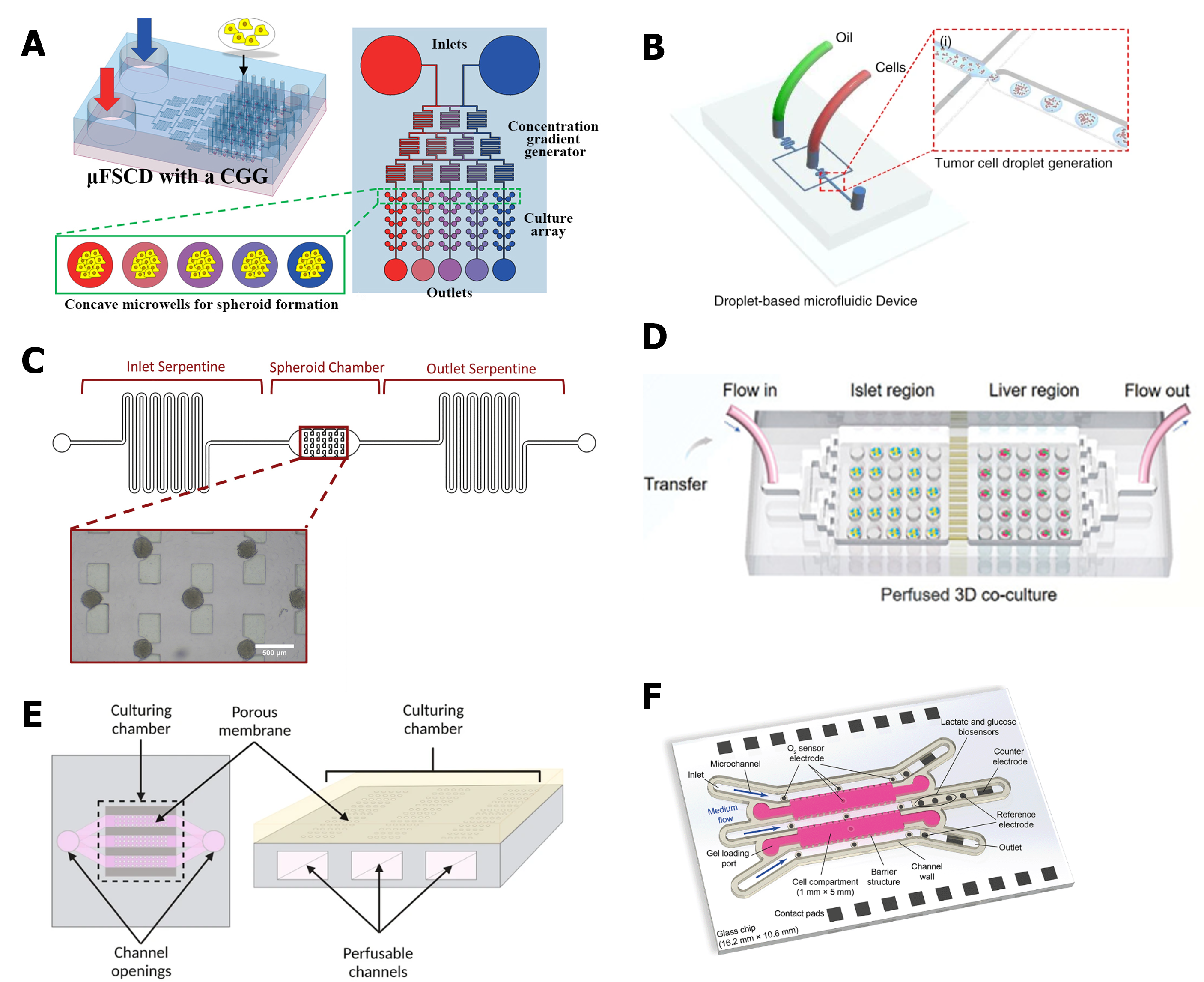
Figure 3 Examples of spheroids- or organoids-on-chip. (A) Microfluidic platform for the generation of chemical gradients for photothermal therapy in spheroids. Figure reprinted with permission from [15]. (B) Platform for the generation of organoids through droplet microfluidics. Image reprinted with permission from17. (C) Microfluidic device capable of spheroid trapping and filtration. Image reprinted with permission from [19]. (D) Microfluidic platform for the co-culture of several replicas of liver and islet organoids. Image reprinted with permission from [22]. (E) Compartmentalized device for the co-culture of kidney organoids and endothelial cells. Image reprinted with permission from [23]. (F) Sensors coupled with a microfluidic device for 3D culture. Image reprinted with permission from [24].
-
Co-culture
The compatmentalization of microfluidic platforms serves multiple purposes, such as hosting and organizing organoids while introducing additional cell types into cell culture models. Notably, endothelial cells represent one of the most intriguing cell types to co-culture with organoids (Figure 3E) [23,25]. To support long-term cultures and more closely mimic tissues, researchers aim to integrate vascularization networks with organoids through the co-culture of endothelial cells. Moreover, the process of embryogenesis is inherently linked to vascular development, indicating the vascularized organoids offer a more accurate replication of organogenesis. However, creating vascularized organoids is challenging for certain organoid types.
For example, while tumor organoids can be co-cultured with tumor cells and endothelial cells in vitro, achieving similar results with organoids derived from pluripotent stem cells (iPSCs) proves more complex. iPSC differentiation protocols rely on specific small molecules introduced at varying concentrations and time points. Incorporating endothelial cells and their media into these cultures may disrupt the expected differentiation outcomes. Despite these challenges, microfluidic devices provide a solution by functioning as mini-bioreactors. They enhance media diffusion through iPSC-derived organoids, preventing the formation of necrotic centers [26].
In addition to endothelial cells, immune cells represent another fascinating cell type to include in organoid models [27]. This especially relevant in tumor models, where studying immune cell reactions to tumors and treatments can yield valuable insights into tumor responses to therapy.
Connections through media channels
Apart from culturing different cell types in the same microfluidic device and/or organoid, different microfluidic platforms or compartments can be connected through media flow. This allows the culture of organoids from different tissues to form a multiorgan system. This system can be used to study the interaction of the different organs with themselves or to test drugs and evaluate the side effects of these compounds in tissues/organs that the drug was not designed to act on [28,29].
-
Integration of sensors
In addition to supporting the production and maintenance of organoids and spheroids, microfluidic platforms offer significant advantages for analyzing these models. Most microfluidic devices are fabricated with transparent materials, enabling researchers to monitor cultures useing microscopy techniques during cultivation or after staining. Furthermore, sensors can be integrated into the platform to track critical culture parameters such as oxygen levels, pH, or temperature (Figure 3F) [24,30]. This real-time monitoring ensures that cultures progress correctly and provides valuable data on cell growth, viability, metabolic activity, and maturation.
For instance, cardiomyocytes and neural cells exhibit electrophysiological activity when they reach a mature state or phenotype. By incorporating multi-electrode arrays into microfluidic platforms, researchers can continuously monitor these activity levels without interrupting the culture. This integration reduces manual handling and minimizes the need for multiple replicates at different time points for activity assessments [31]. Consequently, microfluidic platforms streamline the analysis process while preserving the integrity of the culture.
References
-
Hofer, M. & Lutolf, M. P. Engineering organoids. Nat Rev Mater 6, 402–420 (2021).
-
Huang, B.-W. & Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. Journal of Controlled Release 270, 246–259 (2018).
-
Velasco, V., Shariati, S. A. & Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst Nanoeng 6, 76 (2020).
-
Fang, G., Chen, Y.-C., Lu, H. & Jin, D. Advances in Spheroids and Organoids on a Chip. Adv Funct Mater 33, 2215043 (2023).
-
Sharf, T. et al. Functional neuronal circuitry and oscillatory dynamics in human brain organoids. Nat Commun 13, 4403 (2022).
-
Kim, H., Kamm, R. D., Vunjak-Novakovic, G. & Wu, J. C. Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell 29, 503–514 (2022).
-
Nounsi, A. et al. Patient-Derived Tumoroid for the Prediction of Radiotherapy and Chemotherapy Responses in Non-Small-Cell Lung Cancer. Biomedicines 11, 1824 (2023).
-
Zhao, Z. et al. Organoids. Nature Reviews Methods Primers 2, 94 (2022).
-
Cho, A.-N. et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun 12, 4730 (2021).
-
Lee, H. N. et al. Effect of biochemical and biomechanical factors on vascularization of kidney organoid-on-a-chip. Nano Converg 8, 35 (2021).
-
Fang, G. et al. Enabling peristalsis of human colon tumor organoids on microfluidic chips. Biofabrication 14, 015006 (2022).
-
Charelli, L. E., Ferreira, J. P. D., Naveira-Cotta, C. P. & Balbino, T. A. Engineering mechanobiology through organoids-on-chip: A strategy to boost therapeutics. J Tissue Eng Regen Med 15, 883–899 (2021).
-
Rifes, P. et al. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat Biotechnol 38, 1265–1273 (2020).
-
Prince, E. et al. Microfluidic Arrays of Breast Tumor Spheroids for Drug Screening and Personalized Cancer Therapies. Adv Healthc Mater 11, 2101085 (2022).
-
Lim, W. & Park, S. A microfluidic spheroid culture device with a concentration gradient generator for high-throughput screening of drug efficacy. Molecules 23, 3355 (2018).
-
Wang, Y., Liu, M., Zhang, Y., Liu, H. & Han, L. Recent methods of droplet microfluidics and their applications in spheroids and organoids. Lab Chip 23, 1080–1096 (2023).
-
Lee, J. M. et al. Generation of tumor spheroids using a droplet-based microfluidic device for photothermal therapy. Microsyst Nanoeng 6, 52 (2020).
-
Jin, B.-J. et al. Microfluidics platform for measurement of volume changes in immobilized intestinal enteroids. Biomicrofluidics 8, (2014).
-
Bourn, M. D. et al. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. Journal of Controlled Release 326, 13–24 (2020).
-
Zhu, Y. et al. In situ generation of human brain organoids on a micropillar array. Lab Chip 17, 2941–2950 (2017).
-
Tao, T. et al. Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 19, 948–958 (2019).
-
Tao, T. et al. Microengineered multi‐organoid system from hiPSCs to recapitulate human liver‐islet axis in normal and type 2 diabetes. Advanced Science 9, 2103495 (2022).
-
Bas-Cristóbal Menéndez, A. et al. Creating a kidney organoid-vasculature interaction model using a novel organ-on-chip system. Sci Rep 12, 20699 (2022).
-
Dornhof, J. et al. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip 22, 225–239 (2022).
-
Schulla, L. S. et al. Development of a Novel Microfluidic Co-culture model to study Organoid Vascularization. bioRxiv 2022.03.25.485813 (2022).
-
Berger, E. et al. Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip 18, 3172–3183 (2018).
-
Zhang, J. et al. Immunotherapy discovery on tumor organoid-on-a-chip platforms that recapitulate the tumor microenvironment. Adv Drug Deliv Rev 187, 114365 (2022).
-
Skardal, A. et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci Rep 7, 8837 (2017).
-
Koning, J. J. et al. A Multi-Organ-on-Chip Approach to Investigate How Oral Exposure to Metals Can Cause Systemic Toxicity Leading to Langerhans Cell Activation in Skin. Frontiers in Toxicology 3, (2022).
-
Zhang, Y. S. et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proceedings of the National Academy of Sciences 114, E2293–E2302 (2017).
-
Spitz, S. et al. Development of a multi-sensor integrated midbrain organoid-on-a-chip platform for studying Parkinson’s disease. bioRxiv 2022.08.19.504522 (2022).

