Researchers from the University of Zaragoza have employed a Beonchip microfluidic device to develop a stratified epithelia-on-a-chip model, showcasing the Be-Transflow as an epithelium-on-chip model for permeability studies.
Figure 1 Be-Transflow device.
Introduction
Replicating the permeability of a tissue barrier is pivotal in evaluating the selective transport of substances, such as chemicals, drugs or cosmetics, through epithelial tissues. The capacity of substances to cross tissue barriers influences their absorption into the bloodstream and effectiveness. Hence, determining the permeability of a substance in the pharmaceutical or cosmetic industry is paramount, especially in toxicological studies.
Organ-on-a-chip technologies excel in the establishment of barrier models in vitro. The miniaturized channels and chambers characteristic of microfluidic devices allow the culture of multiple cell types and recreation of the functionality of the tissue unit, as well as facilitate culture medium extraction in different time points. Be-Transflow allows air liquid interface (ALI) culture of external epithelium such as cornea or skin, while allowing the performance of diffusion studies.
Main findings
Researchers achieved the stratified skin epithelium in this work by culturing an immortalized human keratinocyte cell line (HaCaT cells) on the Be-Transflow platform for 14 days. To create the air-liquid interface, they removed the medium on top of the monolayer four days after seeding. Subsequently, they nurtured the cells using the medium circulating in the bottom channel.
To assess the layer’s viability, researchers performed calcein staining, which revealed homogeneous viability values. Furthermore, the Be-Transflow platform supported the extraction of the developed epithelial layer for histological protocols. For instance, haematoxylin-eosin staining confirmed the formation of a stratified epithelium (Figure 2A). Through immunohistochemistry, researchers verified the presence of the three epidermal layers by detecting the expression of cytokeratin-2, -10, and -14 (Figure 2B-D).
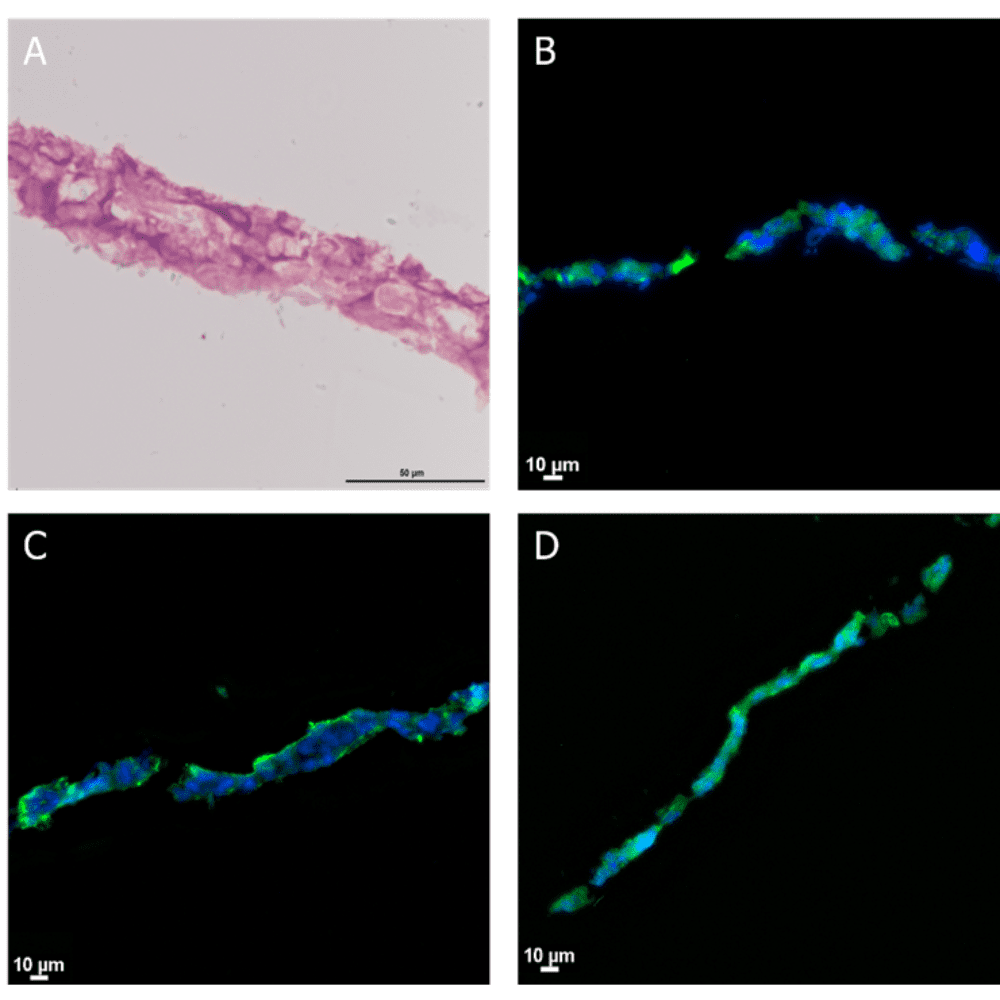
Figure 2 Stratified epithelia of HaCaT cells after 14 days of culture. (A) Haematoxylin-eosin staining. Immunostaining of Cytokeratin 2 (B), Cytokeratin 10 (C) and Cytokeratin 14 (D) in green. Nuclei staining with DAPI (blue).
Permeability assays
Researchers next evaluated whether the epithelia-on-a-chip model could determine the permeability coefficient of different molecules. To achieve this, they assessed the permeability of fluorescein over 4 hours. The results demonstrated a linear slope in the permeation curve, enabling the calculation of fluorescein’s permeability coefficient. Importantly, the obtained values closely matched those found in human corneal endothelium.
In addition, researchers quantified the permeability coefficients of FITC-labeled dextran with varying molecular weights. The model successfully correlated the Stokes radius of the compounds with their permeability coefficients based on the Stokes-Einstein equation. This finding is particularly significant because it allows researchers to estimate a molecule’s Stokes radius by determining its permeability coefficient—or vice versa.
Subsequently, the team used the epithelia-on-a-chip model to determine the Stokes radius of gold nanoparticles via their permeability coefficients. They fabricated nanoparticles of different sizes and evaluated their permeation coefficients in the same manner as the dextran molecules. From these values, researchers calculated the Stokes radius of the particles, obtaining results comparable to those derived from Transmission Electron Microscopy.
Overall, this study highlights the Be-Transflow device’s valuable role as an epithelium-on-a-chip platform for permeability studies. Notably, the model proved effective in determining Stokes radius as it relates to molecular or particle diffusion. Furthermore, this system demonstrates its versatility for studying epithelia from various organs, including skin, kidney, intestine, and lung.
If you wish to know more about this study, please check the full version of the paper here.

