Summary
Here we provide a comprehensive and brief overview of a scientific publication detailing the developmental journey of the Be-Gradient Barrier-Free device. In it, we elucidate its intricate design process and rigorous testing procedures in a collaborative effort between Beonchip and the University of Zaragoza[1]. Furthermore, researchers at IBEC highlighted the pivotal role of this device by generating a blood-brain barrier (BBB)-on-chip model, underscoring its efficacy and potential in the field.
Read the full article here.
Introduction
Chemical gradients play a critical role in numerous physiological processes, governing various cellular functions within living organisms[2,3]. However, conventional in vitro models, such as 2D or 3D cultures, often struggle to replicate these gradients accurately. Consequently, our understanding of how these gradients influence healthy, injured, or diseased tissues is severely limited. Organ-on-chip technology offers a solution to this challenge. Organ-on-a-chip platforms mimic the complex microenvironment of living organisms through interconnected chambers and channels. These microfluidic platforms allow researchers to create controlled gradients that replicate chemical environments influencing cellular processes. Consequently, they offer precise tools for studying how chemical gradients affect tissue and cellular regulation.[4,5]. Thus, organ-on-a-chip models mark a significant advancement in biomedical research, providing unparalleled insights into cellular behavior.
Many gradient-based chips incorporate physical barriers, often in the form of pillars, to confine cell-embedded hydrogels within specific regions[6,7]. However, these barriers can introduce challenges, particularly in maintaining consistent gradient profiles near the physical confines. Furthermore, such barriers can impede direct cell-to-cell contact, crucial for fostering optimal co-culture conditions. This less-than-ideal cellular interaction may compromise the formation of tight junctions, which are fundamental for establishing a functional barrier within the model system.
Be-Gradient Barrier-Free device
The Be-Gradient Barrier-Free device represents a significant advancement over its predecessor, the original Be-Gradient chip. In this updated iteration, the physical barrier that traditionally separated the central chamber from the two lateral channels was eliminated. Instead, the central chamber has been ingeniously treated to facilitate the containment of a hydrogel, enabling the culture of distinct cell types within three separate compartments. This innovative design not only streamlines the experimental setup but also promotes the generation of precise chemical gradients essential for studying various cellular responses and interactions.
In addition to their capability to replicate gradients, Be-Gradient Barrier-Free devices offer compelling advantages for barrier models. Facilitating direct cellular interactions between different cell types, these devices enable the development of barriers that closely mimic native physiological conditions. Of particular significance is the blood-brain barrier (BBB), a remarkable feature of the human body renowned for its high selectivity[8]. This barrier plays a crucial role in supplying essential nutrients and oxygen to the brain while effectively shielding it from potentially harmful pathogens and molecules.
The notable selectivity of the blood-brain barrier presents a challenging obstacle in the development of therapeutic agents for central nervous system diseases, as these agents must successfully traverse the barrier to exert their intended effects. Therefore, creating accurate and reliable models to replicate this barrier is imperative for assessing the efficacy of novel therapeutics.
The development of Be-Gradient Barrier-Free
Starting from the previous version of the Be-Gradient device, the research detailed in this latest publication focuses on the device’s evolution to eliminate physical barriers between a central chamber and two lateral channels. Alongside the existing commercially available design, researchers explored alternative geometries for the central chamber to assess hydrogel confinement.
Results revealed that all variations of the central chamber effectively contained a collagen hydrogel (Figure 1). Indeed, the separation between the lateral channels (stained in red) and the hydrogel on the central chamber (stained in green) is visible. The hydrogel’s confinement within the current Be-Gradient configuration was evaluated under both static and dynamic conditions, yielding favourable outcomes. The alternative geometries evaluated (Figure 1A-D) could also confine the hydrogel, validating the method of fabrication developed.
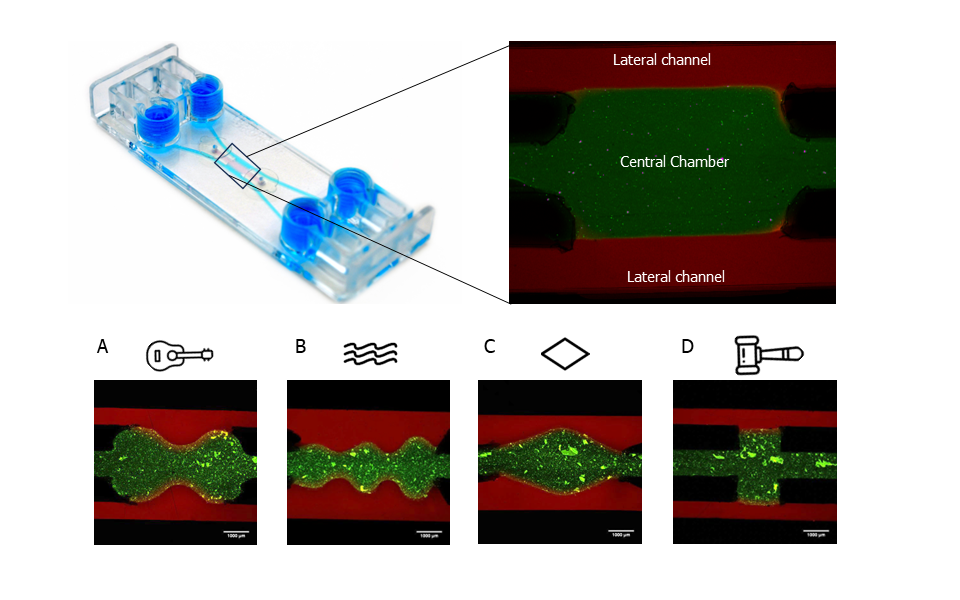
Figure 1: Confinement of a hydrogel in the central chamber of Be-Gradient Barrier-Free device. Other tested geometries: (A) guitar; (B) waves; (C) diamond and (D) hammer. Scale bars 1mm. Images sourced from the original paper[1].
Utilizing finite element analysis with FEniCS, computer simulations were employed to modulate the shear stress and oxygen concentration in both versions of the Be-Gradient chip. In the previous version, the presence of pillars impeded fluid flow, resulting in diminished shear stress within the chamber. Conversely, in the barrier-free iteration, the interface between the chamber and channel exhibited higher levels of shear stress and oxygen concentrations.
Be-Gradient device as a BBB-on-a-chip model
The BBB represents one of the most complex barriers to replicate in vitro due to its intricate composition, comprising multiple cell types closely interacting with each other and the extracellular matrix. The diverse array of cell types and matrix components within this barrier presents specific requirements that must be meticulously addressed to recreate its physiological conditions accurately. As such, achieving successful co-culture of these components within an in vitro model continues to elude researchers.
Researchers at IBEC used the BE-Gradient device as the platform for a BBB model. They cultured human astrocytes and pericytes within a fibrin hydrogel in the central chamber, while human endothelial cells were cultured in one of the lateral channels to replicate the natural BBB environment (Figure 2A, B).
Viability studies conducted after 7 days demonstrated high viability rates across all co-cultured cell types, translated from the Calcein AM activated by the cells ( Figure 2C, D). Thereby confirming the viability of the cells in this experimental setup.
Integrity and performance of the BBB-on-chip
The presence of tight junctions between endothelial cells is imperative for the integrity and performance of the BBB. In this investigation, researchers examined the expression of tight junction markers between endothelial cells, employing staining for ZO-1 and VE-cadherin (Figure 2E). The results revealed the presence of tight junctions between these cells, underscoring the fidelity of the BBB model.
Subsequently, permeability assays were executed using fluorescent tracers of varying molecular weight. On the seventh day, NaFl (376 Da) and D70 (70,000 Da) were introduced into the endothelial channel to assess the blood-brain barrier’s permeability characteristics, with fluorescence imaging conducted in the neuronal region. The findings from the BBB-on-a-chip experiment demonstrated size-dependent exclusion, with smaller molecules (NaFl: 3.9 x 10-5 cm s-1) traversing the barrier more rapidly than larger counterparts (Dextran 70: 1.92 x 10-5 cm s-1). Notably, the permeability values observed in our developed BBB model were consistent with those documented in prior literature concerning microfabricated 3D models.
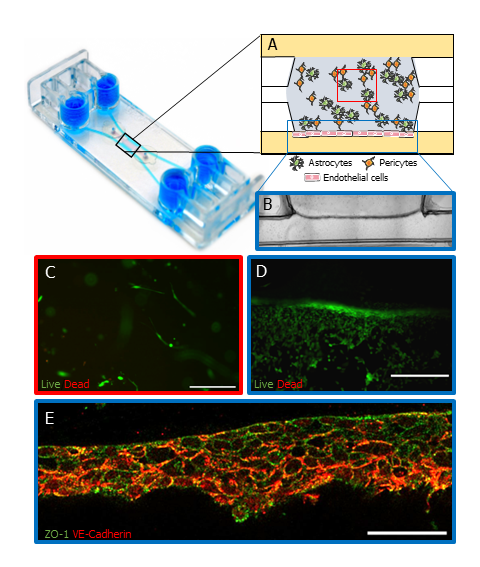
Figure 2: BBB-on-a-chip using BE-Gradient Barrier-Free device. (A) Schematic of human pericytes and astrocytes co-cultured in a fibrin hydrogel in the central chamber of the device while human endothelial cells were cultured on the lateral channel. (B) Brightfield image of the astrocytes and pericytes in the central chamber and endothelial cells in the lateral channel (blue rectangle in A). (C) Confocal images of cell viability assay on the central chamber (red square of A) and the lateral channel (D, blue rectangle in A) on day 7. Scale bars 100 µm and 250 µm respectively. (E) Immunofluorescence of tight junction markers, ZO-1 (green) and VE-Cadherin (red) (blue rectangle in A). Scale bar 50 µm. Cell representation on schematic A used images from Servier Medical Art, licensed under Creative Commons Attribution 4.0 Unported License. Images B,C D and E were sourced from the original paper1.
Conclusions
The findings presented in this study indicate that the manufacturing method of our new Be-Gradient device enables the replication of various tissue-like geometries and the generation of specific shear stress patterns, enhancing the accuracy of experimental models. Moreover, the results illustrate that this approach effectively maintains the confinement of hydrogels in the central chamber over time, even under flow conditions. Through this consistent fabrication process, the devices can create uniform gradients within the central chamber due to the absence of physical barriers, facilitating continuous diffusion through the hydrogel. Lastly, biological validation confirms the functionality of pillarless microfluidic devices in generating a BBB, thus affirming that the described technology meets the necessary criteria for developing in vitro 3D organ-on-a-chip models.
Read the full article here.
More information about the Be-Gradient Barrier-Free can be found here.
Also, check our technical note about hydrogel confinement and diffusion profile in the Be-Gradient Barrier-Free device.
Bibliography
1. Olaizola-Rodrigo, C. et al. Tuneable hydrogel patterns in pillarless microfluidic devices. Lab Chip (2024) doi:10.1039/d3lc01082a.
2. Keenan, T. M. & Folch, A. Biomolecular gradients in cell culture systems. Lab Chip 8, 34–57 (2007).
3. Oudin, M. J. & Weaver, V. M. Physical and Chemical Gradients in the Tumor Microenvironment Regulate Tumor Cell Invasion, Migration, and Metastasis. Cold Spring Harb. Symp. Quant. Biol. 81, 189–205 (2016).
4. Ahmed, M. A. M. & Nagelkerke, A. Current developments in modelling the tumour microenvironment in vitro: Incorporation of biochemical and physical gradients. Organs-on-a-Chip 3, 100012 (2021).
5. Somaweera, H., Ibraguimov, A. & Pappas, D. A review of chemical gradient systems for cell analysis. Anal. Chim. Acta 907, 7–17 (2016).
6. Uzel, S. G. M. et al. Simultaneous or Sequential Orthogonal Gradient Formation in a 3D Cell Culture Microfluidic Platform. Small 12, 612–622 (2016).
7. Dornhof, J. et al. Microfluidic organ-on-chip system for multi-analyte monitoring of metabolites in 3D cell cultures. Lab Chip 22, 225–239 (2022).
8. Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-brain barrier: From physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).

