In this technical note, we focus on Be-Gradient Barrier-Free applications. In particular, the confinement of an hydrogel of different concentrations and their diffusion profiles.
Introduction
Our new design Be-Gradient Barrier-Free is a device designed for 3D culture where a central chamber is linked with two fluidic lateral channels (Figure 1). The innovation we present is the absence of any physical barrier between the central chamber and the lateral channels.
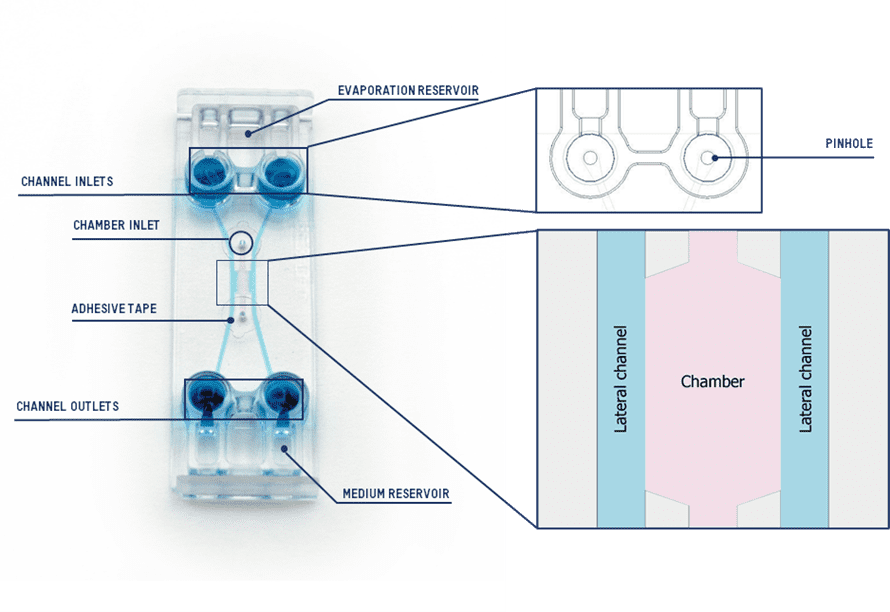
Figure 1 Be-Gradient Barrier-Free device with the description of each section.
The Be-Gradient Barrier-Free device is specifically designed for the application of electrochemical gradients, as well as for creating barrier and vasculature models with 3D cell cultures. Moreover, it is compatible with various types of optical microscopy, including inverted phase contrast, confocal, and fluorescence techniques. For barrier and vasculature models, the lateral channels simulate blood vessels, providing an environment where researchers can culture endothelial cells. Additionally, the device enables the creation of chemical gradients by adjusting the concentration of a specific element between the channels on either side of the central chamber.
In many in vitro biological models, researchers commonly use various hydrogels such as Matrigel, collagen, and fibrin. Collagen, in particular, is the most abundant protein in the human body and plays a critical role in maintaining tissue integrity and elasticity. It provides structural support and acts as an anchor, facilitating cell migration and survival(1). Importantly, the concentration of collagen varies across tissues and biological models, depending on the organ and the specific pathophysiology being studied. For this reason, it is essential to validate that the Be-Gradient Barrier-Free device can accommodate different hydrogel concentrations, including collagen.
In this study, we evaluated the spatial distribution of collagen hydrogels with varying concentrations in the central chamber. Furthermore, we confirmed the proper confinement of the hydrogel within the chamber. To complement this analysis, we also examined the diffusion profile of rhodamine across three different collagen hydrogel concentrations.
Methodology
Filling and handling
Before seeding:
- Prewarm the device in the incubator overnight to avoid the appearance of air bubbles.
- The central culture chamber must be completely dry to confine the hydrogel in it.
- Prepare the collagen type I solutions following the protocol described in González-Lana et al.(2). For this assay, we used 5; 1; 2 and 4 mg/mL. Keep the solution at 4°C until the time of seeding.
- Add FluoSpheres™ (Invitrogen™, Fisher Scientific, 10513463) to the collagen solution in a ratio of 100:1.
- Pipette 10 µL of the solution and slowly inject it through the inlet of the central chamber until filling it midway (Figure 2 A. 1-3). Then, pipette the rest of the solution from the outlet of the chamber until the chamber is fully filled (Figure 2 A. 4-6). When changing the tip from the inlet to the outlet to finish injecting the solution, keep pressing the pipette plunger so air does not enter the pipette tip. The central chamber can also be completely filled by one side only if performed slowly and carefully (Figure 2 B).
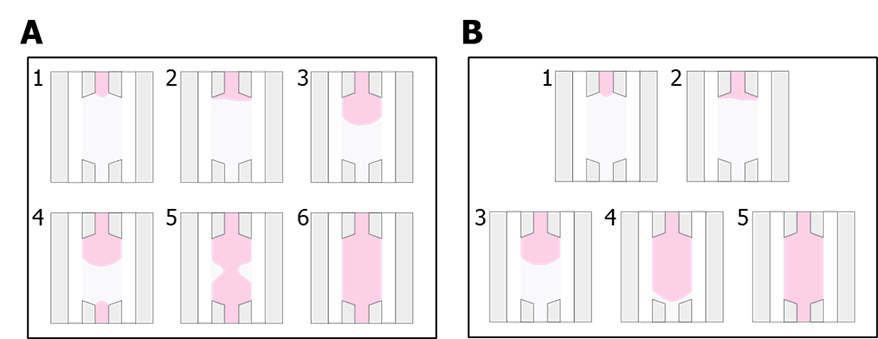
Figure 2 Different options to seed the central chamber of the BE-Gradient Barrier-Free. (A) Filling the chamber from both inlet and outlet. (B) Filling the chamber from the inlet only.
4. Flip the chip upwards and downwards every 5 minutes several times for homogeneous 3D distribution during the polymerization of the hydrogel. Keep the device at 37 °C, for a total of 15 minutes.
Disclaimer: this step is dependent on the hydrogel. Check manufacture’s recommendations for polymerization and apply to this protocol.
5. Seal the central chamber inlet and outlet with the adhesive provided. Make sure that the surface is clean and dry to assure a proper adhesion. Do not seal the central chamber before the hydrogel is fully polymerized, otherwise the hydrogel can leak into the lateral channels when the tape is placed.
6. Within the lateral channels, place an aqueous solution with rhodamine (1 µg/mL; MW 01; Sigma-Aldrich, R6626). To introduce the solution in the channels, first pipette around 250 mL in the inlet well of the channel and gently aspirate from the outlet, ensuring that the tip of the pipette is vertical and in the pinhole. After the channel is filled with the medium, there’s no need to keep aspirating; it will flow naturally from the inlet to the outlet.
See the tutorial video here.
Assessment of the fluorescence
To evaluate the hydrogel spatial distribution, we recommend using a fluorescence or confocal microscope capable of 3D reconstruction.
- Place the device with the fluorescent hydrogel in the central chamber when the hydrogel is polymerized but the channels are still empty (step 5 from the previous section).
- Acquire images on the microscope at various z-planes throughout the chamber’s height to ensure the hydrogel is fully filling the whole chamber.
- After, fill the channels with the fluorescent solution and repeat the image acquisition, preferably in the same zone or similar.
Results
One of the most important features of Be-Gradient Barrier-Free is the ability to contain a hydrogel in the central chamber while having direct contact to two lateral channels. This feature aims to create a closer environment for cell-cell interaction in barrier models or gradient formation.
Hydrogel confinement
It is important to ensure the suitability of this device with hydrogels of different consistencies. Collagen is extensively used in in vitro models both as coating and as 3D matrices. The consistency of this material is different if the concentration is as low as 0.5 mg/mL or as high as 4 mg/mL. The former is more fluid and the latter more viscous and denser.
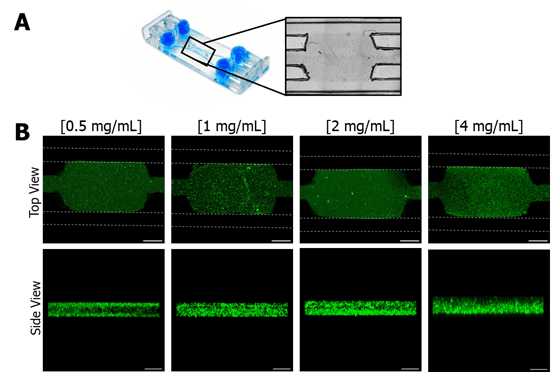
Figure 3 Confinement of different collagen concentrations in Be-Gradient Barrier-Free central chamber. (A) Brightfield image of the area of the device with the hydrogel confined in the central chamber and the lateral channels. (B) Confocal images of different collagen concentrations (0.5, 1, 2 and 4 mg/mL) loaded to the chamber of BE-Gradient Barrier-Free device. The first line shows the top view, and the second line shows the side view of the devices. The dotted lines in green represent the lateral channel positions. Scale bar: 1 mm (first row); 200 µm (second row).
We tested four different concentrations of collagen—0.5, 1, 2, and 4 mg/mL—by staining the hydrogel with fluorescent particles and loading it into the device’s central chamber. Using confocal microscopy, they observed that all concentrations successfully filled the central chamber without spreading into the lateral channels (Figure 3, Top View). A lateral view assessment confirmed that the hydrogel reached the entire chamber, ensuring proper confinement. These findings highlight the device’s adaptability to hydrogels with varying concentrations, mechanical properties, stiffness, and pore size.
Diffusion profile
Next, we assessed the diffusion profile across hydrogels with different concentrations (0.5, 1, and 2 mg/mL) by perfusing a rhodamine solution into one lateral channel (Figure 4). They observed that the lowest concentration (0.5 mg/mL) allowed faster fluorescence diffusion, completely traversing the central chamber within 25 minutes and generating a fluorescence gradient (Figure 4C). In contrast, hydrogels at 1 and 2 mg/mL concentrations exhibited similar diffusion behavior, with fluorescence reaching only halfway across the chamber.
After 15 minutes, all concentrations showed red fluorescence in the center of the chamber, indicating that the rhodamine solution had reached this region (Figure 4). Researchers extrapolated that if both lateral channels were filled, it would take approximately 15 minutes for the solution to fully diffuse across the chamber.
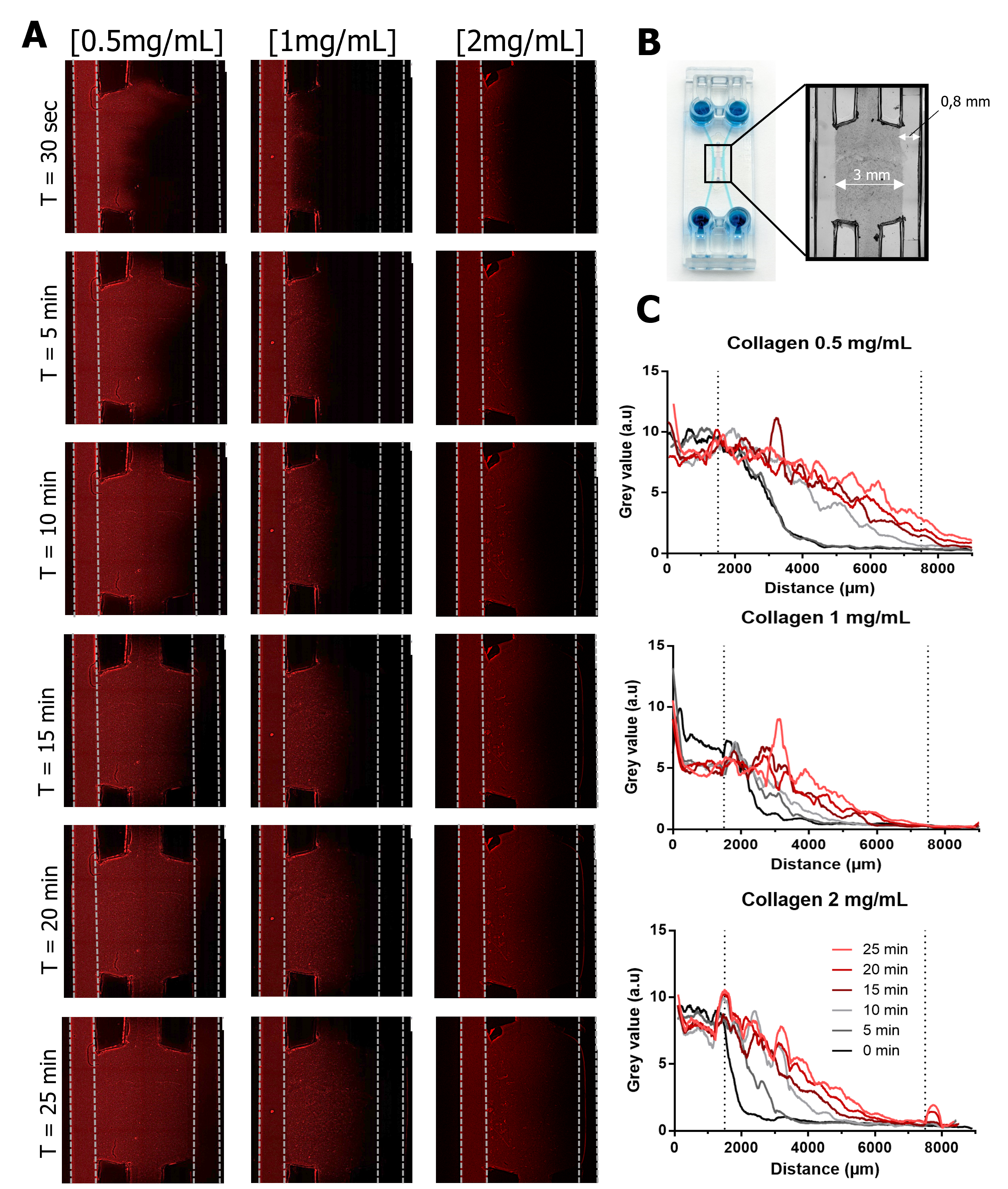
Figure 4 Diffusion test. (A) Fluorescence images of rhodamine diffusion from the left channel into the central chamber using three different collagen concentrations (0.5; 1 and 2 mg/mL) in the central chamber over 25 minutes. Scale bar: 1 mm. (B) Area of the device that was measured. (C) Plot profiles of the images acquired.
Understanding the diffusion rate in hydrogel is essential for determining the time required for media to reach seeded cells. Additionally, it helps predict the time needed for solutions used in protocols, such as immunostaining, to penetrate effectively. Importantly, the time of diffusion varies depending on the type of hydrogel and its concentration. This is because the hydrogel’s porosity directly influences the speed at which liquid diffuses from the channels to the chamber.
Moreover, the number of seeded cells significantly affects the diffusion velocity through the central chamber. Denser cell cultures slow down the diffusion process, whereas less densely seeded cultures allow for faster diffusion. These factors collectively highlight the importance of tailoring hydrogel properties and cell densities to optimize experimental conditions.
Conclusions
Beonchip recently launched the Be-Gradient Barrier-Free device, introducing a versatile tool for advanced research applications. This device features a central chamber flanked by two lateral channels in direct contact. Researchers designed it specifically for 3D culture and the development of electrochemical gradients and barrier models.
Collagen, a widely used extracellular matrix protein in 3D culture, plays a key role in these studies. In this technical note, researchers loaded the device with collagen hydrogels at varying concentrations. They demonstrated that the hydrogels remained confined within the central chamber, regardless of the concentration.
To further validate the system, researchers introduced a rhodamine solution and observed its diffusion behavior. The results confirmed effective diffusion across the hydrogel without compromising the confinement of the solution. Additionally, the study revealed the diffusion profile, offering valuable insights into how substances traverse the hydrogel matrix.
Bibliography
- Sung, K. E. et al. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials 30, 4833–4841 (2009).
- González-Lana, S. et al. Surface modifications of COP-based microfluidic devices for improved immobilisation of hydrogel proteins: long-term 3D culture with contractile cell types and ischaemia model. Lab Chip 23, 2434–2446 (2023).

