Introduction
The intestine harbors a commensal microbiome that plays a crucial role in its physiology and functionality. Notably, some of the microorganisms residing in the intestine require anaerobic conditions to survive. [1] For the small intestine, oxygen levels have been reported as 4.1–4.5% in the intestinal lumen. [5] Therefore, achieving these anaerobic intestinal environment and oxygen controled conditions in vitro generally requires specialized techniques and equipment with inert gases, such as nitrogen and argon.
On the other hand, in the field of microfluidics for cell culture, most platforms for intestine-on-a-chip models are made of PDMS (polydimethylsiloxane). [2] However, PDMS is highly permeable to gases and water vapor and also tends to nonspecifically adsorb lipophilic compounds. [3,4] Hence, using new materials with low or no gas permeability represents an alternative for simulating anaerobic environment by effectively controlling oxygen concentration.
Beonchip uses cyclic olefin polymer (COP) for the manufacturing of its devices. This material is biocompatible and with a refractive index close to glass. Aditionally, It has very low gas permeability, allowing for precise control of gas concentrations within the devices. Given these characteristics, COP microfluidic devices are ideal for recreating the intestinal environment. Furthermore, they enable accurate oxygen control.
Based on these data, we aimed to create a physiologically relevant anaerobic intestinal environment in the Be-Doubleflow device. To achieve this, we measured oxygen levels in the culture medium before entering and after exiting the microfluidic channel. The channel contained an intestinal epithelial monolayer under different flow rates.
Materials
Microfluidic device
The Be-Doubleflow (Fig. 1) chip consists of two fluidic channels separated by a porous membrane, allowing the exchange of substances and factors between the cell types in both compartments. Moreover, these channels feature threaded inlets and outlets designed for connection to perfusion systems, ensuring culture medium renewal. Consequently, the system generates tangential forces that directly influence cellular behavior. Specifically, these tangential forces arise from the flow and speed of the culture medium moving through the channels and are known as shear forces or shear stress.
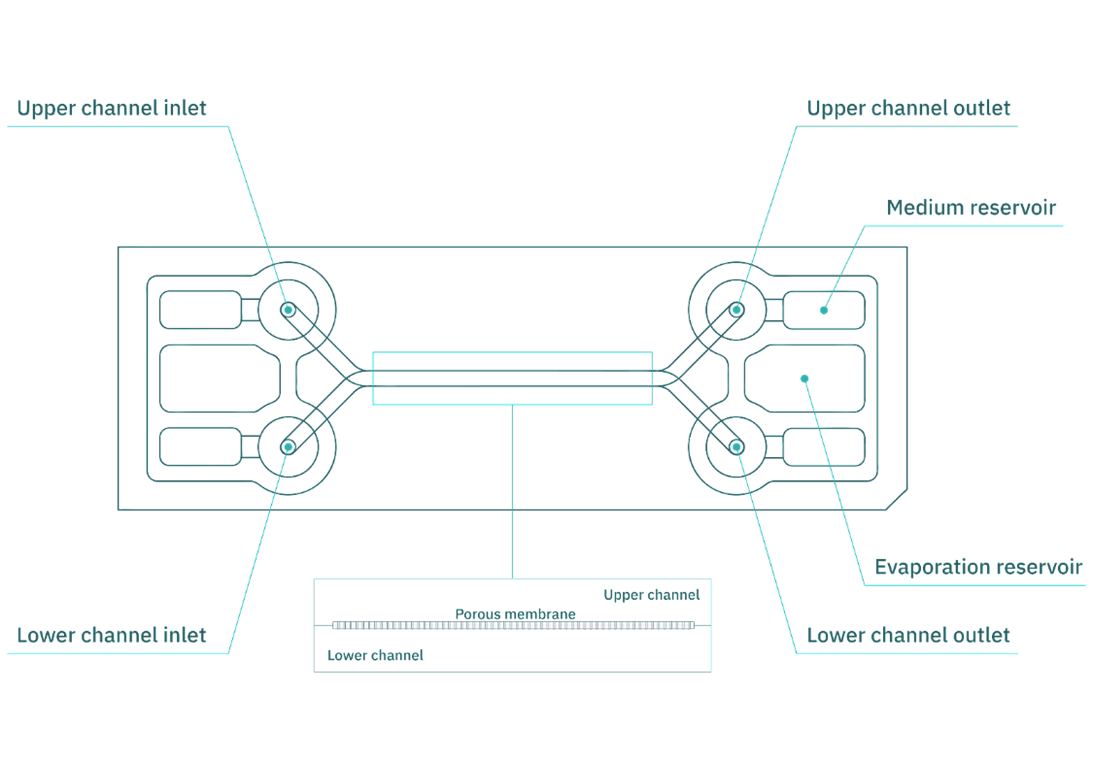
Figure 1. Be-Doubleflow microfluidic device used for intestinal epithelium in vitro culture.
Microfluidic set-up and oxygen sensors
Microfluidic cirtuit elements
A syringe pump (Infusetek) perfused the culture medium into the device, ensuring a continuous flow. As the medium moves through the system, it exerts tangential mechanical stimulation (shear stress) on the cell surface while delivering essential nutrients and gases. In addition, the tubing used (FEP/Tygon), and other components of the system have very low gas permeability. Consequently, this setup ensured that the sensor readings reflected the oxygen content in the liquid without any losses from the circuit.
To monitor oxygen levels, off-chip fluidic oxygen sensors (FTC-SU-PSt7-10-YOP-S) were incorporated at both the inlet and outlet of the fluidic system. We used the Measurement Studio 2 program to make the measurements. The PC connects to the OXY-4 ST transmitter, linking the polymer optical fiber (POF-FTC-L2.5-1ST) to the oxygen flow sensor in the microfluidic circuit (Fig. 2).
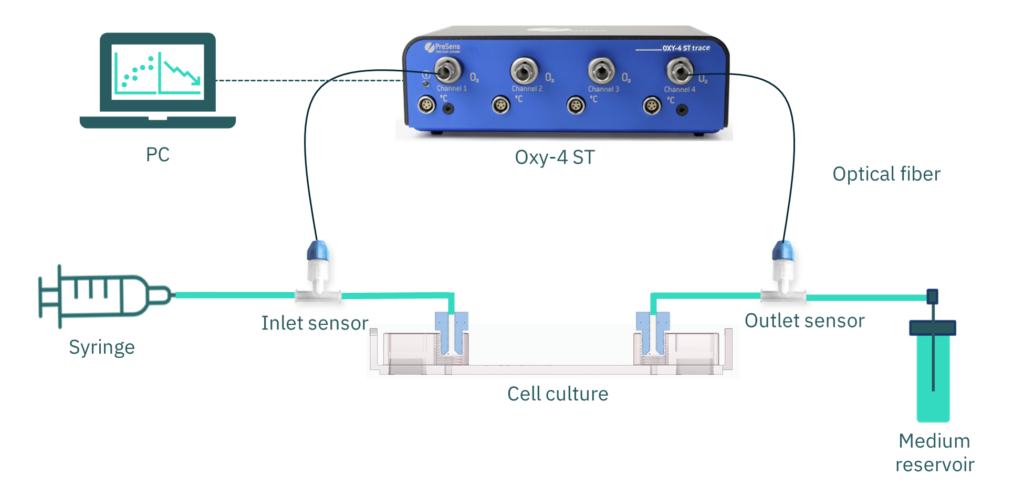
Figure 2. Diagram of the experimental elements. Be-Doubleflow microfluidic device is connected to a syringe perfusion set-up. Oxygen flow sensors (PreSens), at the inlet and outlet of the cell culture channel, connect through optical fiber to a transmitter (OXY-4 ST). A computer receives the signal by a USB connection with the transmitter.
Oxygen sensors
Flow oxygen sensors (FTC-SU-PSt7-10-YOP-S) offer the following advantages:
• They do not invade the fluidic channel for cell culture. Thus, they provide an easy connection to external tubing. In addition, this design is compatible with both flexible and semi-rigid tubing.
• Being sterile and disposable, they eliminate the need for a cleaning protocol.
• They allow gas control in both the upper (epithelial) and lower (endothelial) channels.
• Additionally, they enable oxygen measurement at both the inlet and outlet of the channel, facilitating real-time monitoring of cellular oxygen consumption.
Before using the sensors in the system, we tested a sodium sulfite (oxygen-free) solution to ensure readings of 0% (Fig. 3).
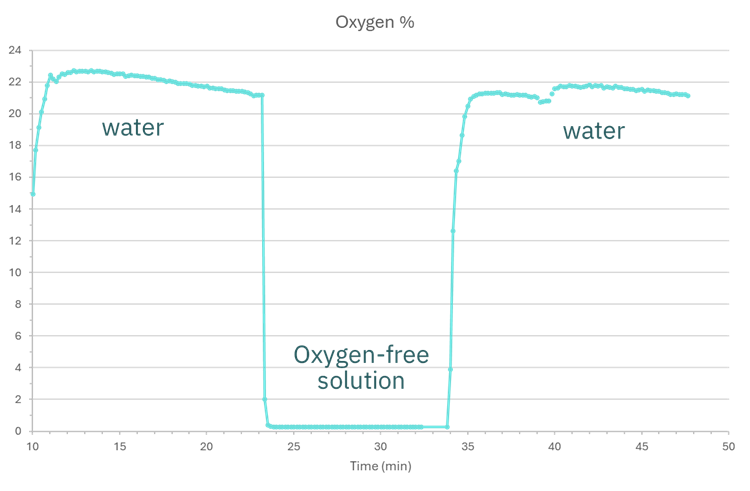
Figure 3. Oxygen values water and oxygen-free solution.
Control of culture conditions for an anaerobic intestinal environment
Cell culture in Be-Doubleflow
We used epithelial cells from the immortalized commercial Caco-2 cell line in a co-culture ratio that simulates small intestine conditions with HT29MTX cells (modified for mucus production) in the channel of the Be-Doubleflow device (Fig. 1).
Firstly, we injected a collagen coating in PBS (0.1 mg/mL) into the channel and incubated for 2 hours at 37°C. Before seeding, the coating solution was gently removed by completely emptying the channel.
Next, a suspension of one million Caco-2 + HT29MTX cells (9:1) in 40 µL of high-glucose DMEM culture medium supplemented with 10% fetal bovine serum and 1% non-essential amino acids was introduced into the microfluidic channel. After 4 hours of static incubation at 37°C, 5% CO₂, we added additional 300 µL of culture medium to fill the medium reservoirs.
Then, we incubated the device for 24 hours (at 37°C, 5% CO₂) on a rocker, allowing for automatic culture medium renewal through gravitational flow. This gentle flow enables the formation of a viable cellular monolayer.
After confirming the formation of the cellular monolayer using optical microscopy, we assembled the device to a syringe pump with connectors, tubing and oxygen sensors. Finally, we perfused the culture medium through the closed system at the previously set flow rates. Along with, we programmed an oxygen measurement every 5 minutes.
Medium flow and shear stress
In order to characterize the oxygen concentration in the culture medium and adjust the flow rate according to oxygen consumption, we applied different shear stress to Be-Doubleflow.
The literature reports that the shear forces exerted on the intestinal epithelium range between 0.002 and 0.08 dyn/cm², with 0.02 dyn/cm² being the most commonly used value by researchers. [6] Thus, shear stress levels of 0.02 dyn/cm² and 0.01 dyn/cm² were applied.
For this purpose, we applied Formula 1, in which the flow rate or velocity corresponding to a given shear stress in a rectangular channel is calculated considering its height, width, and the viscosity of the liquid.
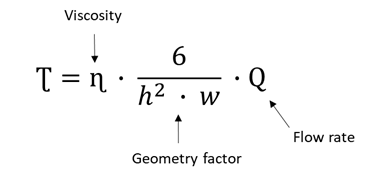
Formula 1. Shear stress (Ʈ) formula in a rectangular channel, where Q is the flow rate, ɳ is the hydrodynamic viscosity of the culture medium (0.0072 dyn*s/cm²)7 and w (0.15 cm) and h (0.0375 cm) are the width and height of the flow channel.
Thus, the flow rates for 0.02 dyn/cm² and 0.01 dyn/cm² are 5.6 and 2.9 µL/min, respectively.
Results
Oxygen consumption vs flow rate
The initial results revealed that after 24 hours of culture under flow conditions, oxygen levels at the channel outlet decreased from initial values of 18–20% to 12–14% with 0.02 dyn/cm². On the other hand, oxygen dropped to 6% with 0.01 dyn/cm² after 12 hours of perfusion (Fig. 4).
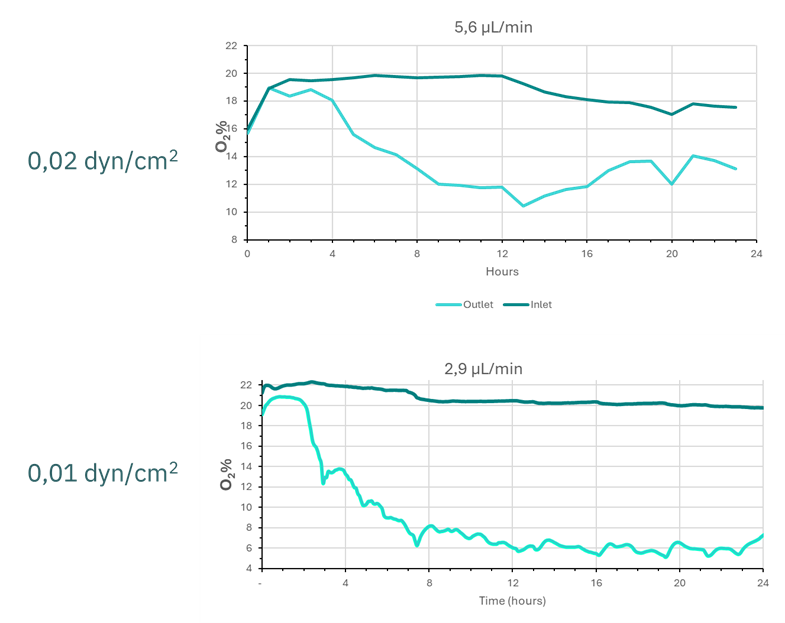
Figure 4. Graphical representation of oxygen values in the culture medium at the inlet (dark line) and outlet (light line) of the epithelial culture channel.
Considering the initial results, the shear stress condition of 0.01 dyn/cm² demonstrated oxygen levels closer to the anaerobic intestinal environment for the lumen and mucosa (4.1–4.8%). As a result, we maintained this condition for three days to evaluate the progression of oxygen consumption.
Figure 5 illustrates the first 8–12 hours, during which the O₂ percentage decreases drastically before stabilizing at values between 5% and 8%.
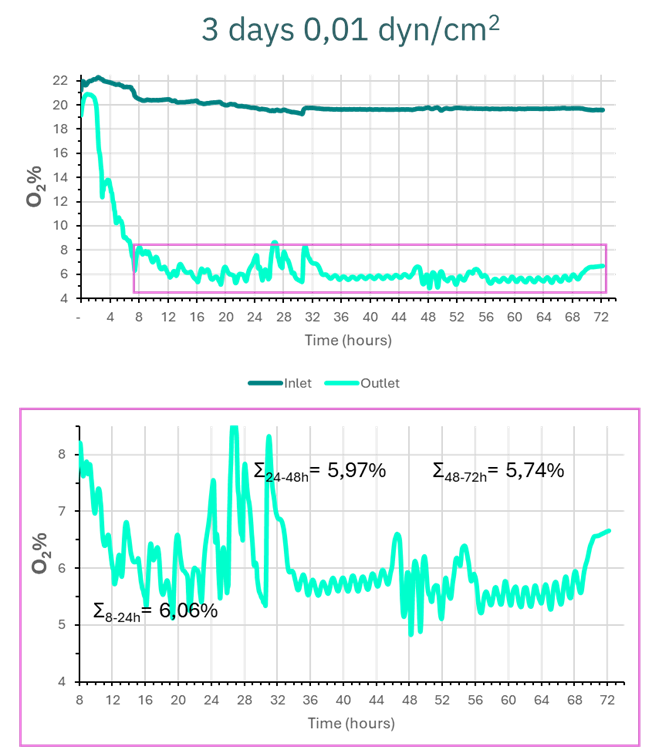
Figure 5. Graphical representation of oxygen values in the culture medium at the inlet and outlet of the epithelial culture channel over 3 days at 0.01 dyn/cm².
By analyzing the values over time intervals, we determined that the average oxygen level at the device outlet was 6.06% from 8 to 24 hours. In the next 24-hour period (24–48 hours), the average value dropped slightly to 5.97%. Finally, in the last period (48–72 hours), the average oxygen level reached 5.74%.
Therefore, we conclude that from 8 hours of culture onward, oxygen levels stabilize between 5–6%, while nutrients are continuously renewed. This setup enables the creation of an anaerobic intestinal environment that accurately mimics physiological conditions in the Be-Doubleflow.
Epithelial integrity and structural maturation
The epithelial monolayer remained intact after three days under 0.01 dyn/cm² (Fig. 6). Moreover, an increase in height was observed, along with the formation of three-dimensional structures such as domes and pseudovilli, indicating epithelial maturation and differentiation.
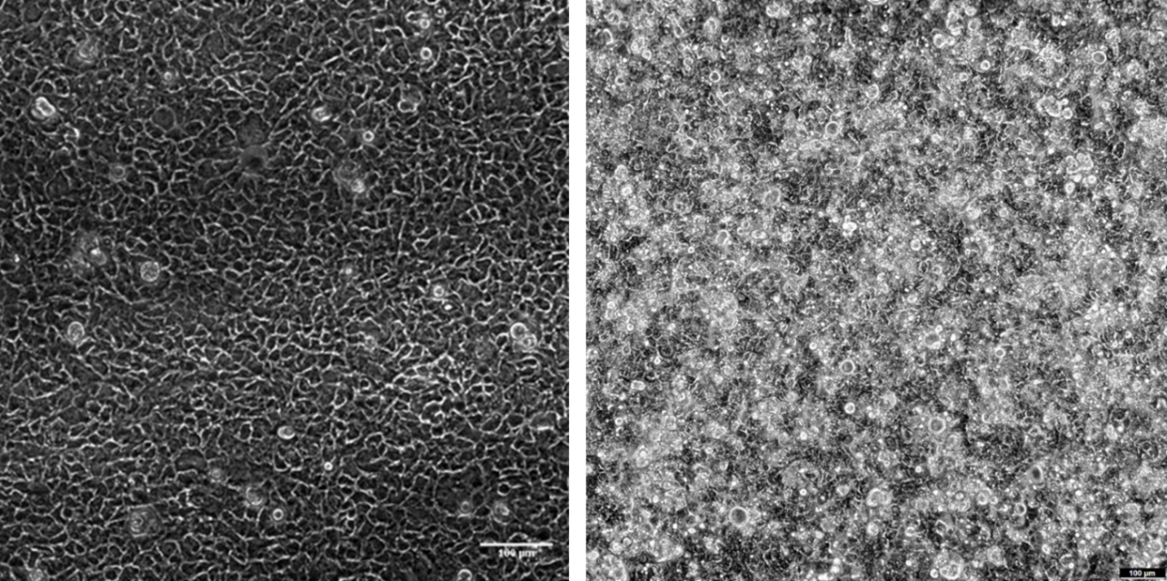
Figure 6. Cell monolayer of the Caco-2 and HT29MTX co-culture after 20 hours of seeding in the device before perfusing the culture medium (left); after 3 days of perfusion (0.01 dyn/cm²; right) in Be-Doubleflow. Phase contrast images. Scale bar: 100 µm.
Conclusions
In conclusion, the flow rate of the culture medium significantly impacts the oxygen concentration consumed by the Caco2+HT29MTX (9:1) co-culture.
A flow rate of 5.6 µL/min (0.02 dyn/cm²) leads to an oxygen consumption of 6-10% (compared to the inlet concentration), which falls outside the oxygen reported in the literature. This indicates that higher flow rates may not replicate the typical oxygen consumption seen in the small intestine. On the other hand, a flow rate of 2.9 µL/min (0.01 dyn/cm²) results in oxygen consumption of 14-15%. This, in turn, averages at 6% oxygen, aligning closely with the reported values for the small intestine. Thus, lower flow rates more accurately simulate physiological conditions.
Hence, the Be-Doubleflow microfluidic device, combined with Presens oxygen sensors, offers an effective platform for studying oxygen levels and creating an anaerobic intestinal environment in vitro.
References
- Ashammakhi, N. et al. Gut-on-a-chip: Current progress and future opportunities. Biomaterials 255, (2020).
- Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019).
- Kim, D. & Herr, A. E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 7, 1–47 (2013).
- Nam, G. et al. Water permeable flow of polydimethylsiloxane controlled by physicochemical treatment. JMST Adv. 1, 41–47 (2019).
- Keeley, T. P. & Mann, G. E. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol. Rev. 99, 161–234 (2019).
- Chi, M. et al. A microfluidic cell culture device (μFCCD) to culture epithelial cells with physiological and morphological properties that mimic those of the human intestine. Biomed. Microdevices 17, 1–10 (2015).
- Poon, C. Measuring the density and viscosity of culture media for optimized computational fluid dynamics analysis of in vitro devices. bioRxiv 2020.08.25.266221 (2020) doi:10.1101/2020.08.25.266221.
Contact
Do you want to know more? Contact us at info@beonchip.com

