Summary
Over the past few years, bioprinting has emerged as a compelling field of study. The field of organ-on-chip has particularly benefited from bioprinting applications, offering novel pathways to enhance in vitro cell culture methods. This technical note explores intriguing possibilities arising from the synergistic integration of these two cutting-edge technologies.
Introduction
3D printing, or additive manufacturing, fabricates three-dimensional objects by depositing successive layers of material until the intended shape is formed. This technique is opposed to traditional subtractive manufacturing where the material is removed from a sold block to create a shape.[1] 3D printing first starts with the creation of the digital model of the object by computer-aided design software and then slicing the digital model into thin horizontal layers. Subsequently, these thin layers are printed sequentially to build the object.
Advantages of this technique
- Design flexibility: 3D printing allows for the fabrication of complex designs and geometries that are challenging or even impossible with traditional manufacturing techniques.
- Cost-effectiveness for prototyping: with this technique prototypes can be manufactured quickly and at a fraction of the cost, allowing for a faster iteration and refinement of designs.
- Reduced waste: since 3D printing is an addictive process, the material deposited is needed only, avoiding the waste material subtractive manufacturing has.
- On-demand manufacturing: with quick and cheaper prototyping, the products can be fabricated on-demand, without the need for large inventories or big storage space/costs.
- Complexity without assembly: with 3D printing most designs can be printed in a single whole piece, without the need to assemble multiple parts after printing, reducing the assembly time and labour, as well as weak points that can arise from the union points of the pieces.[2]
Bioprinting is described as the controlled deposition of cells and biomaterial. It is encompassed inside the technology of 3D printing and was adapted to the construction of biology models. This technique allows the deposit of different biomaterials and cell lines in the same construct, translating into more complex tissue constructs with human-like features such as tissue functions.
Bioprinting on a microfluidic platform
Bioprinting technology has the potential to streamline microfabrication procedures, potentially tackling the challenges of throughput and reproducibility encountered by conventional organ-on-chip systems.[3]
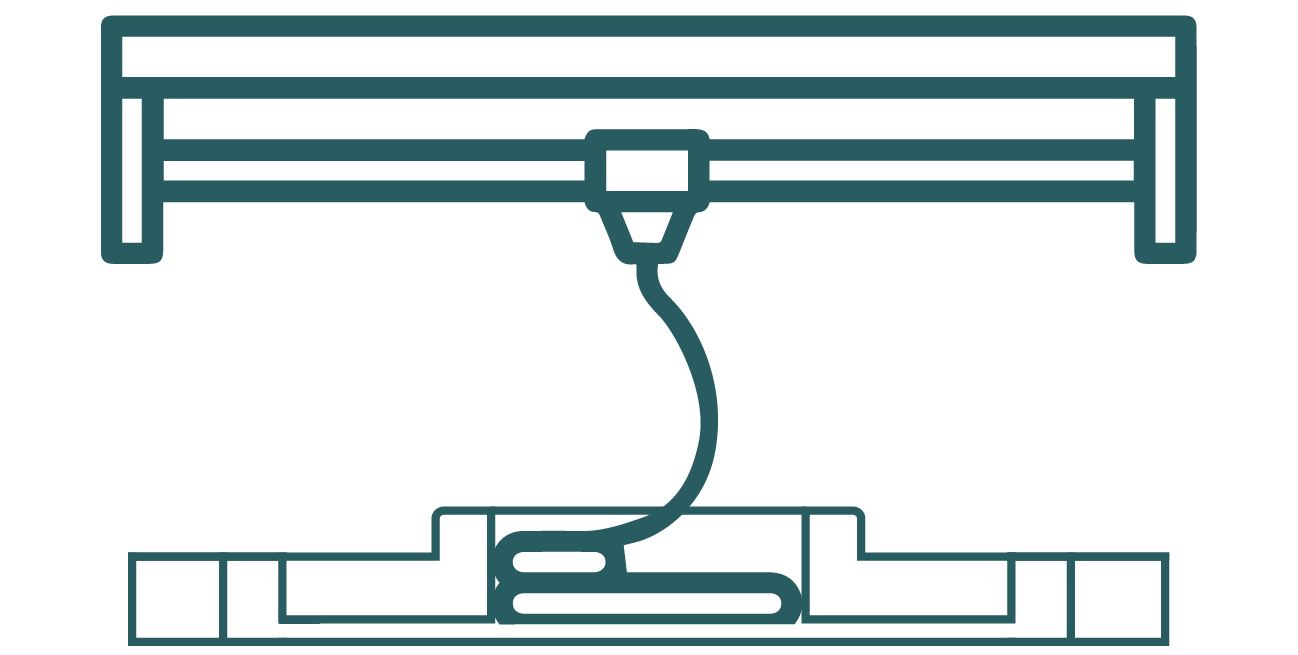
Figure 1: Bioprinting inside a microfluidic platform.
Applications of this technology
Develop a microenvironment that mimics the heterogeneity of cells and extracellular components found in nature
Achieving an extracellular matrix-like heterogeneous composition of biomaterials in microfluidic devices has been a significant challenge. Therefore, consistently printing biomaterial formulations directly onto a microfluidic chip could revolutionize the creation of biomimetic microenvironments. Customizing bioink compositions makes it possible to produce heterogeneous tissue constructs with various cell types and substrates, employing multiple exchangeable printheads with different materials.
Achieve consistent cell printing and patterning directly within the microfluidic devices
Cells are typically manually seeded inside a microfluidic chamber, a generally slow process. Therefore, using a bioprinter for direct multiple-cell printing and/or patterning would enable high-throughput, high-precision production of consistent tissue constructs with minimal human intervention.
Generate intricate 3D microstructures
The versatility of bioprinting modes enables the deposition of biomaterial-cell spheroid-tissue strands into complex, free-form 3D structures on the chip, allowing the creation of cell culture models specifically designed for studying cell-cell and cell-matrix interactions.
Simulate barriers and interfaces within the vascular system
Achieving successful vascularization has been a significant challenge in creating functional tissues for in vitro models. Advanced microfluidic-assisted bioprinting techniques allow for the generation of multiscale hydrogel-based flow networks that closely mimic the form and function of real vessels. Bioprinting these vascular networks can enable fluid flow, facilitating nutrient transport, gas exchange, and waste removal.
Printing of microfluidic devices
Traditionally, soft lithography, photolithography, and etching techniques have been widely used to fabricate OOC devices, but these methods have significant limitations that hinder development and innovation in microfluidic applications. These limitations include 1) the necessity for numerous repeated processing steps; 2) the inability to create continuously curved structures and complex geometries; 3) long lead times and high labour requirements; 4) issues with reproducibility, dimensional accuracy, and surface quality; and 5) the need for separate cleanroom facilities and skilled users. Consequently, a rapid prototyping technique is required to overcome the limitations of these conventional methods.[4]
3D printing of microfluidic devices presents an innovative approach to fabricating complex and precise structures with various applications in biotechnology, medical research, and chemical analysis. This technology allows for the creation of micro-scale channels, chambers, and intricate networks that are essential for manipulating small volumes of fluids in a controlled environment.
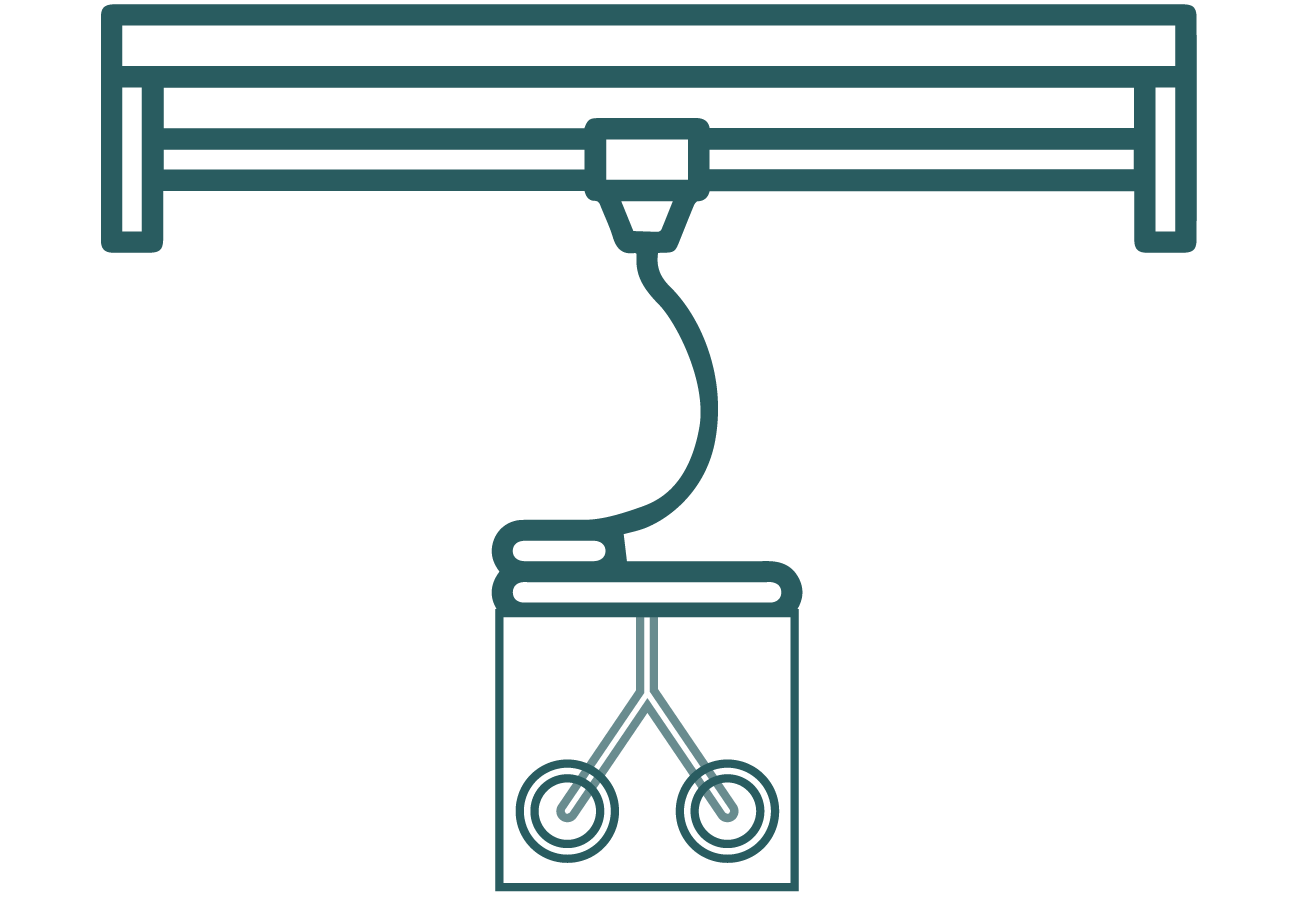
Figure 2: 3D printing the microfluidic device.
Suitable Printing Techniques
Several printing techniques are suitable for fabricating 3D microfluidic devices:
Stereolithography (SLA):
In stereolithography, a specific pattern of UV light or laser is directed over a liquid photopolymer, causing the polymer to cure and form three-dimensional structures. This patterning is achieved using digital micromirror arrays, which allow for the creation of highly complex, flexible, and scalable structures.[5] Unlike laser-assisted bioprinting, stereolithography employs a layer-by-layer process that enhances speed and resolution, achieving less than 25 µm resolutions. This technique supports the use of high-precision polymers such as acrylics and epoxies, although it is limited by the range of materials available and requires intense UV exposure and extensive post-processing times.[6]
- Resolution: High, typically around 20-100 µm.
- Advantages: Offers excellent precision and surface finish. It can create complex geometries and is compatible with a range of materials, including biocompatible resins.
- Disadvantages: Limited to photopolymerizable materials, which may have restricted mechanical properties and biocompatibility.
Digital Light Processing (DLP):
Digital light processing is a layer-by-layer technique, similar to SLA but distinguished by the method of light projection onto the photosensitive material using digital light mirrors.[7, 8]
- Resolution: Comparable to SLA, with resolutions down to 10 µm.
- Advantages: Faster than SLA as it cures entire layers at once. High precision and good material properties.
- Disadvantages: Similar material limitations as SLA, with potential issues in scaling up productions.
Fused Deposition Modelling (FDM):
Fused deposition modelling works by extruding thermoplastic filament through a heated nozzle, layer by layer, to build the desired structure.[9] The viscoelastic properties of the processed materials are crucial at each stage of the FDM process: predicting the printability of the molten material during extrusion and ensuring a continuous flow through the nozzle; regulating the deposition process of the moulded part and ensuring layer adhesion during the subsequent consolidation phase.
- Resolution: Lower than SLA and DLP, typically around 50-200 µm.
- Advantages: Cost-effective, wide range of materials, including thermoplastics like PLA and ABS. Suitable for rapid prototyping.
- Disadvantages: Lower resolution and surface finish, less suitable for highly detailed or complex microstructures.
Two-Photon Polymerization (2PP):
Two-photon polymerization is a specialized form of stereolithography that enables the creation of three-dimensional structures with resolutions beyond the diffraction limit. This technology is often utilized for fabricating microfluidic devices and replicating physiological microenvironments in vitro due to its high precision, achieving resolutions of less than 100 nm. However, the high resolution of 2PP results in extended fabrication times, making it impractical for constructing larger tissue analogues. Additionally, 2PP is restricted to using photosensitive polymers from the microelectronics industry, which typically have lower biocompatibility.[6]
- Resolution: Extremely high, down to 100 µm.
- Advantages: Can create nanoscale features with exceptional precision, ideal for intricate microfluidic structures.
- Disadvantages: Expensive equipment and slow process, limiting its use for large-scale production.
3D printing provides significant advantages for microfluidics, standing out when compared to traditional methods like photolithography and soft lithography. Its design flexibility allows researchers to create highly complex and customized microfluidic structures that traditional techniques cannot achieve. Additionally, it enables rapid prototyping, facilitating quick iterations and accelerating development cycles to advance research in microfluidics. During fabrication, 3D printing directly integrates components like valves, mixers, and sensors, reducing assembly time and minimizing potential failures. Furthermore, its compatibility with a wide range of materials, including biocompatible and transparent options, enhances versatility for specific applications. As a result, 3D printing becomes an efficient and innovative choice for fabricating microfluidic platforms.
Disadvantages of 3D printing for chip manufacturing
Although 3D printing offers many advantages, it also has drawbacks compared to traditional methods. For instance, resolution limitations can arise; while some techniques achieve high resolution, methods like Fused Deposition Modeling often lack the precision needed for specific microfluidic applications. Moreover, material restrictions pose challenges, as some printing techniques are confined to materials lacking the required mechanical or chemical properties for certain uses. Transparency is another limitation, as most available materials are opaque, complicating cell culture visualization in microfluidic devices.
The high cost of high-resolution printers and specialized materials limits accessibility for some researchers and small-scale productions. Additionally, scalability remains challenging, as printing time and costs create significant barriers to large-scale production. However, ongoing advancements in printing technology are steadily addressing these issues. Finally, the finished products often lack the refined appearance of those made with injection molding, potentially impacting the aesthetic and functional quality of microfluidic platforms.
Microfluidic platforms in bioprinting
Lack of Precision on 3D bioprinting
The anatomical arrangement of human tissues at the micro, meso, and macro scales to form complex 3D hierarchical structures is essential for the proper function of individual tissues and organs, and consequently, the organism as a whole.[10] However, a significant limitation of current 3D bioprinting technologies is the lack of microscale precision and control over cellular composition or arrangement, particularly when using multiple cell types or achieving single-cell resolution during the printing process.[11] This loss of microscale precision during mesoscale fabrication hampers the organization of different cell types within a tissue assembly, limiting the reproducibility and application of artificial tissues.[12] To create artificial biological tissues that function as effectively as natural ones, it is crucial to position specific cell types in multiscale structures with hierarchical features that replicate the natural microscale tissue environment. Therefore, a multi-material, bottom-up biofabrication approach would be highly advantageous.
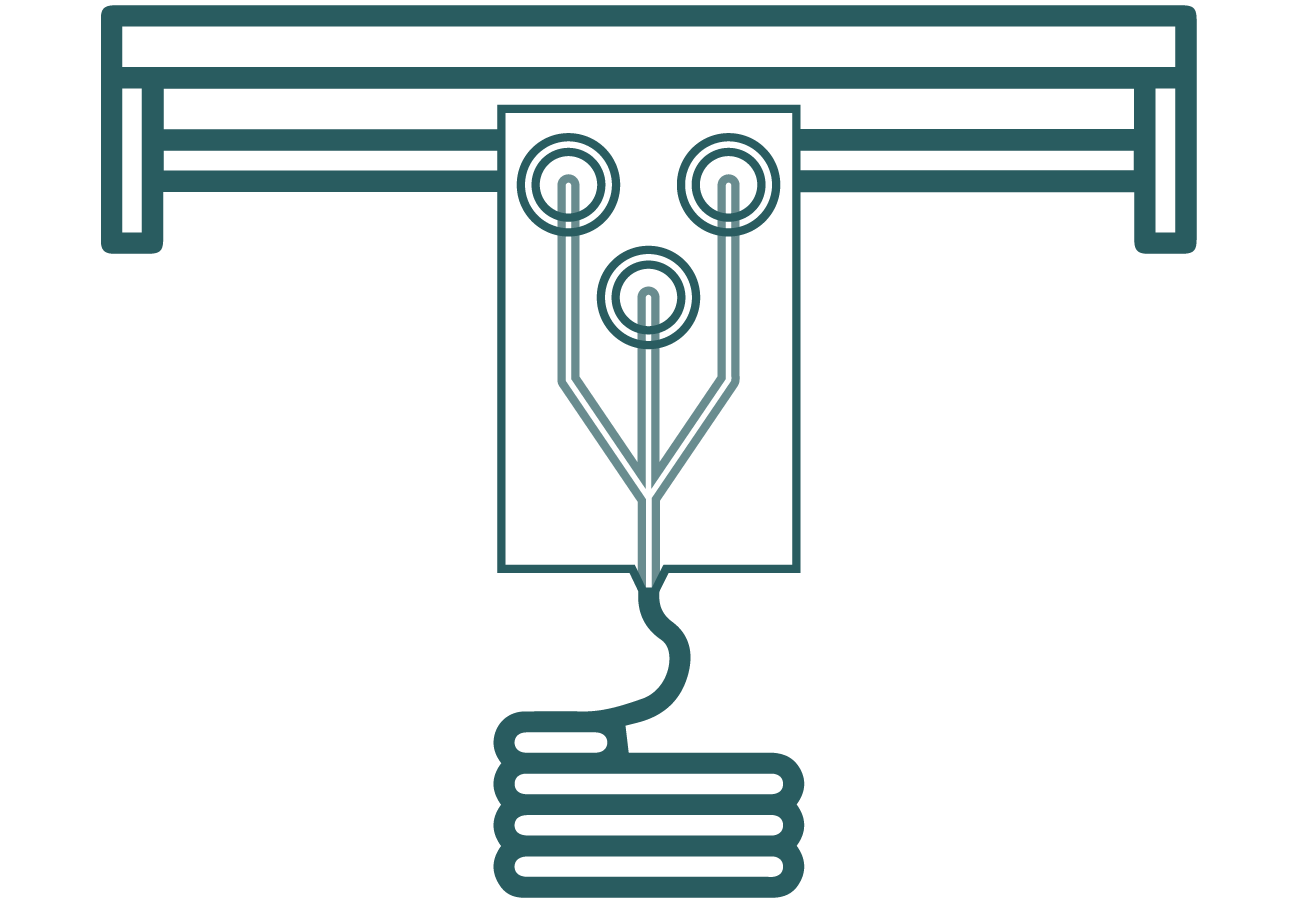
Figure 3: Microfluidic platform as the printing head for bioprinting.
Incorporation of microfluidic devices as printing noozles to improve precision
To enhance microscale precision, microfluidic platforms can be integrated into biofabrication methods to manipulate cells and biomaterials effectively. By leveraging the geometric constraints of microscale fluids, where forces like viscosity and surface tension dominate, laminar flow enables precise patterning. Controlled fluid streams carrying biomolecules, cells, organisms, or chemicals can create structures such as cell-laden droplets, microfibers, or cell sheets.[11] Additionally, microfluidic devices support diverse fluidic mechanisms like focusing, concentrating, mixing, and separating, enhancing structural precision.
Furthermore, microfluidic environments with multiple inlets improve printing processes by enhancing cell viability, guiding cell differentiation, and enabling smooth material transitions. Unlike traditional bioprinting, microfluidic biofabrication keeps constructs within the chip instead of extruding them. However, adapting the nozzle to include microfluidic chips allows seamless integration with existing 3D bioprinting platforms. This integration enables multi-material printing with greater control over material concentration, positioning, and composition. Microfluidic-enhanced 3D bioprinting (MF3D) shows promise for creating organoids that better mimic the structural and functional integrity of natural tissues. For instance, vascularized hollow tissues can be printed as cell-laden, multi-layer filaments to form blood vessels. These are interwoven with cardiac cells, printed as droplets within a hydrogel scaffold, to fabricate functional cardiac tissue.
In summary, this note describes the three most used bioprinting applications in organ-on-chip technology. In case you are interested in learning more or need a microfluidic device that can be adapted for bioprinting applications, contact us!
Bibliography
- Thakar, C. M. et al. 3d Printing: Basic principles and applications. Mater. Today Proc. 51, 842–849 (2022).
- Lu, B., Li, D. & Tian, X. Development Trends in Additive Manufacturing and 3D Printing. Engineering 1, 085–089 (2015).
- Yu, F. & Choudhury, D. Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 24, 1248–1257 (2019).
- Tabatabaei Rezaei, N. et al. Recent Advances in Organ-on-Chips Integrated with Bioprinting Technologies for Drug Screening. Adv. Healthc. Mater. 12, 1–22 (2023).
- Carnero, B., Bao-Varela, C., Gómez-Varela, A. I., Álvarez, E. & Flores-Arias, M. T. Microfluidic devices manufacturing with a stereolithographic printer for biological applications. Mater. Sci. Eng. C 129, 112388 (2021).
- Rothbauer, M. et al. Recent Advances in Additive Manufacturing and 3D Bioprinting for Organs-On-A-Chip and Microphysiological Systems. Frontiers in Bioengineering and Biotechnology vol. 10 (2022).
- Amini, A., Guijt, R. M., Themelis, T., De Vos, J. & Eeltink, S. Recent developments in digital light processing 3D-printing techniques for microfluidic analytical devices. J. Chromatogr. A 1692, 463842 (2023).
- Luo, Z. et al. Digital light processing 3D printing for microfluidic chips with enhanced resolution via dosing- and zoning-controlled vat photopolymerization. Microsystems Nanoeng. 9, 103 (2023).
- Acierno, D. & Patti, A. Fused Deposition Modelling (FDM) of Thermoplastic-Based Filaments: Process and Rheological Properties—An Overview. Materials (Basel). 16, (2023).
- Vijayavenkataraman, S., Yan, W.-C., Lu, W. F., Wang, C.-H. & Fuh, J. Y. H. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 132, 296–332 (2018).
- Richard, C., Richard, C., Neild, A., Cadarso, V. J. & Cadarso, V. J. The emerging role of microfluidics in multi-material 3D bioprinting. Lab Chip 20, 2044–2056 (2020).
- Marti-Figueroa, C. R. & Ashton, R. S. The case for applying tissue engineering methodologies to instruct human organoid morphogenesis. Acta Biomater. 54, 35–44 (2017).

