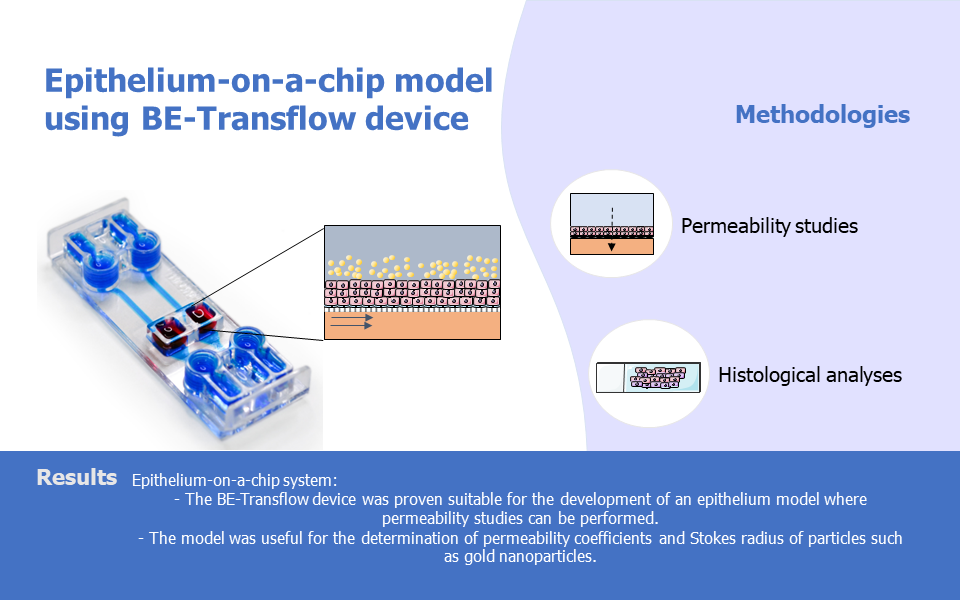
Researchers from the University of Zaragoza have employed the Be-Transflow device to develop a stratified epithelia-on-a-chip model, highlighting this device as a useful platform for permeability studies.
Replicating the permeability of a tissue barrier is pivotal in evaluating the selective transport of substances, such as chemicals, drugs or cosmetics, through epithelial tissues. The capacity of substances to cross tissue barriers influences their absorption into the bloodstream and effectiveness. Hence, determining the permeability of a substance in the pharmaceutical or cosmetic industry is paramount, especially in toxicological studies. Here, we review a scientific publication of the TME Lab, in which the team lead by Jesus Ciriza uses the BE-Transflow as a novel platform for permeability studies.
Article link: https://www.tandfonline.com/doi/full/10.1080/21691401.2023.2253534

Replicating the permeability of a tissue barrier is pivotal in evaluating the selective transport of substances, such as chemicals, drugs or cosmetics, through epithelial tissues. The capacity of substances to cross tissue barriers influences their absorption into the bloodstream and effectiveness. Hence, determining the permeability of a substance in the pharmaceutical or cosmetic industry is paramount, especially in toxicological studies.
Organ-on-a-chip technologies excel in the establishment of barrier models in vitro. The miniaturized channels and chambers characteristic of microfluidic devices allow the culture of multiple cell types and recreation of the functionality of the tissue unit, as well as facilitate culture medium extraction in different time points. Be-Transflow allows air liquid interface (ALI) culture of external epithelium such as cornea or skin, while allowing the performance of diffusion studies.
The stratified skin epithelium used in this work, was achieved by culturing an immortalised human keratinocyte cell line (HaCaT cells) on the well of the Be-Transflow platform for 14 days. Four days after seeding, the medium on top of the monolayer was removed to create the air-liquid interface. Then, the cells were nurtured through the medium circulating on the bottom channel. The viability of the layer formed was assessed through calcein staining, showing homogeneous viability values. The Be-Transflow platform was also compatible with the extraction of the layer epithelium developed to perform histological protocols such as haematoxylin-eosin staining, which showed a stratified epithelium (Figure 1A). The three epidermal layers were confirmed by the expression of cytokeratin-2, -10 and -14, evaluated through immunohistochemistry (Figure 1 B-D).

Figure 1: Stratified epithelia of HaCaT cells after 14 days of culture. (A) Haematoxylin-eosin staining. Immunostaining of Cytokeratin 2 (B), Cytokeratin 10 (C) and Cytokeratin 14 (D) in green. Nuclei staining with DAPI (blue).
The next step was to evaluate if the epithelia-on-a-chip model could determine the permeability coefficient of different molecules. To do so, the permeability of fluorescein was evaluated for 4h and results demonstrated a linear slope of the permeation curve, which allowed the permeability coefficient of this molecule and the values obtained were similar to the ones found in human corneal endothelium. In addition, FITC-labelled dextran of different molecular weights was used for the quantification of their permeability coefficient. The model was able to correlate the Stokes radius of the compounds and their permeability coefficient according to the Stokes-Einstein equation. This result is particularly valuable since it allows the estimation of the Stokes radius of a molecule by determining its permeability coefficient or vice-versa.
Lastly, the authors used the epithelial-on-a-chip model to determine the Strokes radius of gold nanoparticles through the permeability coefficient. Nanoparticles of different sizes were fabricated, and their permeation coefficient was evaluated similarly to the dextran molecules before. From the values obtained, researchers could calculate the Stokes radius of the particles which resulted in values similar to the ones obtained from Transmission Electron Microscopy.
Overall, this paper demonstrates the valuable role of the Be-Transflow device as an epithelium-on-a-chip platform for permeability studies, particularly in determining Stokes radius related to the diffusion of molecules or particles. This model extends their applicability to epithelia found in organs such as skin, kidney, intestine, or lung.
If you wish to know more about this study, please check the full version of the paper here.

Recent Comments